In the intricate tapestry of subatomic physics, a nuanced understanding of forces governing the interactions between particles is paramount. Among these forces, the concept of binding energy plays a pivotal role, particularly in the realm of atomic and nuclear physics. The fascinating question arises: Is binding energy synonymous with the strong nuclear force? To navigate this inquiry, one must elucidate the interplay between these two pivotal concepts, their definitions, and their implications for the structure of matter.
Binding energy, fundamentally, is the energy required to disassemble a system of particles into separate constituents. In nuclear physics, this manifests as the energy needed to separate nucleons—protons and neutrons—within an atomic nucleus. When nucleons are brought together to form a nucleus, they undergo a release of energy, indicative of a stable configuration. This energy release, often quantified in units of mega-electronvolts (MeV), signifies the binding energy of the nucleus, rendering it a compelling subject of study.
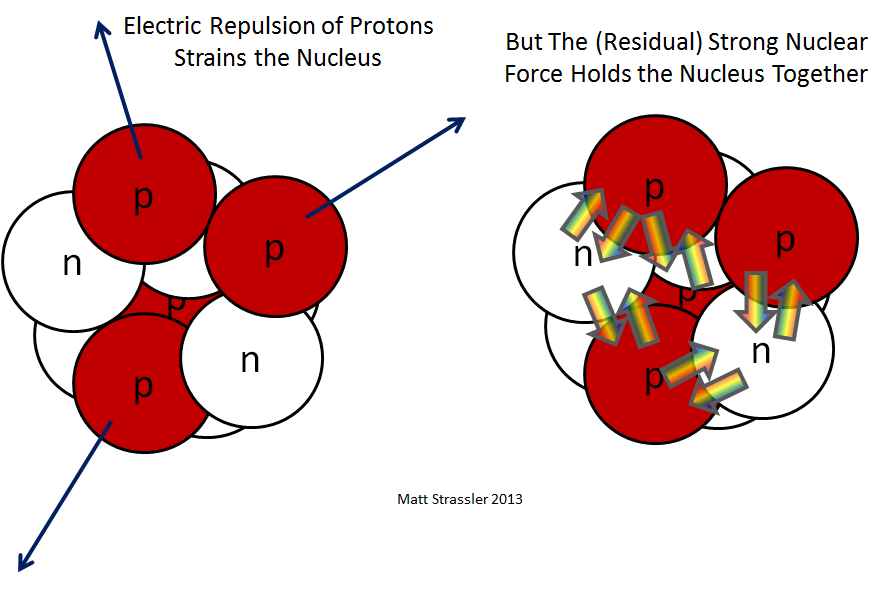
On the other hand, the strong nuclear force is the fundamental interaction responsible for binding these nucleons together within the nucleus. It operates at incredibly short ranges—approximately 1 femtometer (10-15 meters)—and is the strongest of the four known fundamental forces, far surpassing electromagnetic and gravitational forces. The strong force arises from the exchange of particles known as gluons, which mediate the interaction between quarks, the fundamental constituents of protons and neutrons. This interaction is characterized by its potency, wherein the force grows stronger as quarks approach one another, ensuring the stability of the nucleus.
The connection between binding energy and the strong nuclear force is profound yet complex. It is tempting to equate binding energy with this formidable force; however, a more nuanced understanding reveals that while binding energy is a consequence of the strong nuclear force, it is not merely a rephrasing of the force itself. Rather, binding energy quantifies the amount of energy released when nucleons become bound in a nucleus, acting as a metric of the strong force’s efficacy in overcoming the electromagnetic repulsion between protons.
A deeper consideration unveils the essence of nuclear stability. The stability of an atomic nucleus hinges upon the balance of the strong nuclear force and the electrostatic repulsion inherent among positively charged protons. Bound nuclei, therefore, reflect a delicate equilibrium—wherein the strong force endeavors to bind nucleons with remarkable intensity against the counteracting forces of repulsion. The measure of this equilibrium is the binding energy. Hence, a nucleus with higher binding energy exhibits greater stability due to an effective strong force that successfully mitigates repulsions.
To further disentangle these concepts, one must explore the significance of mass-energy equivalence, as articulated by Einstein’s renowned equation, E=mc². The binding energy is intrinsically associated with the mass defect of a nucleus, which represents the difference in mass between its constituent nucleons when isolated and when they are collectively bound in the nucleus. This mass defect reveals that the binding energy is effectively stored in the form of mass—the larger the energy released during binding, the greater the mass defect. Thus, the few MeV that signify binding energies translate directly into the stability and the physical manifestations of these interactions.
The implications of binding energy extend beyond the confines of nuclear stability; they reverberate through astrophysics and cosmology. In stellar nucleosynthesis, the processes governing the formation of elements within stars are predicated upon the interactions driven by the strong nuclear force. Heavier elements, forged in the cores of stars, exhibit diverse binding energies, which dictate their formation pathways and abundance in the universe. It is the interplay between the binding energy and the strong force that engenders the creative processes of our cosmos, from the hydrogen that fuels stars to the complex elements that constitute planets.
Moreover, the study of binding energy and the strong nuclear force finds practical applications in contemporary technology. Nuclear fission and fusion, pivotal processes in energy generation, are predicated on the principles governing binding energy. In fission, the splitting of heavy nuclei releases energy due to the resultant increase in binding energies of the products, while fusion combines light nuclei, where significant energy is released as well. Understanding these energies allows for advancements in energy production, medical applications in radiotherapy, and even the quest for sustainable hydrogen fusion.
In conclusion, while binding energy and the strong nuclear force are intimately interlinked, they embody distinct concepts that deserve careful consideration. Binding energy is a measure of stability derived from the formidable strong nuclear force, representing the energy dynamics at play within atomic nuclei. The fascination lies not merely in their definitions, but in their profound implications for the universe—shaping everything from the formation of elements to the functioning of modern technology. Thus, the inquiry into whether binding energy is the strong nuclear force invites a deeper exploration into the fundamental interactions that govern the subatomic world, illuminating the intricate mechanisms that weave together the very fabric of matter itself.