Isotopes form a captivating aspect of atomic physics, intriguing both scientists and laypersons alike. Their unique behavior and distinctive nature delve into the intricate world of subatomic particles, providing insight into a fundamental structure of matter. This article explores the definition of isotopes, their formation, and the implications of their distinct properties, thereby unraveling the complexities inherent in the subatomic realm.
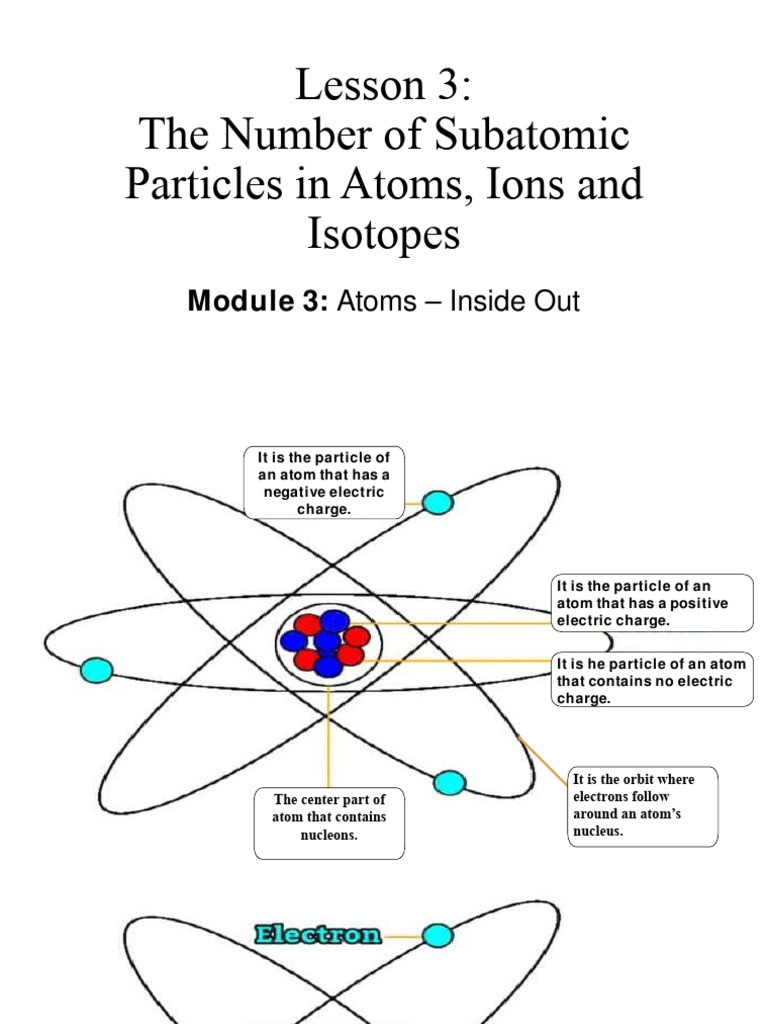
At the core of atomic theory lies the understanding that atoms are composed of subatomic particles—protons, neutrons, and electrons. Protons and neutrons reside in the nucleus, a dense central region, while electrons orbit this nucleus in defined energy levels. The chemical identity of an element is determined by the number of protons it possesses, known as its atomic number. However, isotopes introduce a fascinating layer of complexity: they manifest as variants of a single element that share the same number of protons but differ in their number of neutrons.
To elucidate, consider carbon as a classic example. Carbon normally possesses six protons and six neutrons, which defines its standard atomic weight of 12. However, carbon exists in several isotopic forms. Two notable isotopes are carbon-12, with six neutrons, and carbon-14, which contains eight neutrons. Despite their differing neutron counts, both isotopes retain the same chemical properties, owing to their identical electronic configurations.
This differentiation in isotopes does not merely serve an academic purpose; it fosters an understanding of natural phenomena. Isotopes play crucial roles in various fields such as geology, archaeology, and medicine. One particularly fascinating application is radiocarbon dating, which utilizes carbon-14 to ascertain the age of ancient organic materials. The predictable decay of carbon-14 over time allows scientists to estimate when an organism died, uncovering the hidden narratives of human history.
Syntactically, isotopes can be denoted in a few formats. A common notation includes the element’s symbol followed by its atomic mass number—this number being the sum of protons and neutrons. For instance, carbon-12 is represented as ¹²C while carbon-14 is depicted as ¹⁴C. Alternatively, isotopes can also be expressed in terms of their nuclear composition, highlighting the neutron-proton ratio. This notation not only serves performative purposes but also emphasizes the fundamental discrepancy in nuclear make-up that defines each isotope’s peculiar characteristics.
In a subatomic context, the variations in neutrons have profound implications for the stability of isotopes. While many isotopes are stable, some are inherently unstable and undergo radioactive decay. This decay occurs as the isotope seeks a more stable configuration, often emitting radiation in the process. This dynamism of unstable isotopes leads to their classification as radioisotopes, found in applications ranging from medical treatments to energy production in nuclear reactors. The study of radioisotopes fascinates scientists due to the stochastic nature of decay processes, introducing concepts such as half-life, which quantifies the rate of decay.
Delving deeper, the isotopic dissimilarity also touches on the broader fields of nuclear physics and chemistry. Isotopes can exhibit variation in mass, leading to different physical properties, such as diffusion rates, reaction kinetics, and boiling points. This isotopic substitution can influence chemical reaction pathways, creating unique isotopic signatures that can be employed in advanced analytical techniques like isotope geochemistry and forensic science.
Moreover, isotopes can illuminate the intricate dance of atomic interactions within stars. The nucleosynthesis of elements is heavily dependent on the fusion of isotopes within stellar environments. Understanding how isotopes are produced during these stellar processes allows physicists to piece together the cosmic mosaic from the formation of stars to galactic dynamics.
Another compelling aspect involves the concept of isotopic ecophysiology, where isotopes serve as indicators of biological pathways and metabolic processes. The examination of stable isotopes in biological specimens can provide insights into dietary habits, migration patterns, and ecological relationships among various organisms. As such, isotopes bridge the gap between physics and biological sciences, enriching our understanding of life’s complexities.
Despite their commonality, isotopes remain an enigmatic and multifaceted topic worthy of exploration. Their unique configurations of subatomic particles not only enrich the field of physics but also provide a diverse array of practical applications that benefit society. From elucidating the secrets of ancient civilizations to revolutionizing modern medicine and energy production, isotopes serve as pivotal tools in the ongoing pursuit of knowledge.
In summary, the study of isotopes—variations of elements distinguished by their neutron counts—opens up a world of fascination rooted in the understanding of subatomic particles. Their implications stretch across multiple scientific domains, revealing the interconnectedness of disciplines such as physics, chemistry, biology, and earth sciences. As our grasp of isotopes evolves, so too does our appreciation for the intricate workings of the universe, where even the smallest of particles play a monumental role in the grand tapestry of existence.