When contemplating the intricate world of chemistry, it is crucial to delineate between the terms “molecule” and “compound.” One might pose the question: Is O2 considered a molecule or a compound? This inquiry opens a Pandora’s box of definitions and implications. The challenge lies in the fundamental understanding of these concepts and their applications within the vast realm of chemical science.
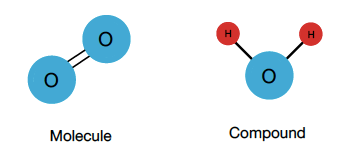
To embark on this exploration, it is imperative to first elucidate the definitions of both terms. A molecule is defined as a group of two or more atoms that are covalently bonded together. These atoms can either be of the same element or of different elements. In contrast, a compound is specifically a molecule that consists of two or more different elements. Thus, a molecule can be a compound if it meets this criterion. However, not all molecules qualify.
O2, or dioxygen, falls under the category of elemental molecules. It is composed entirely of two oxygen atoms bonded together. Consequently, the absence of any different elemental atoms raises an intriguing point for discussion: Can a substance be classified as both a molecule and a compound?
Understanding this dilemma requires a deeper dive into the definitions that underlie these terms. The formation of O2 begins with two oxygen atoms sharing electrons, a quintessential depiction of covalent bonding. Covalent bonds are characterized by the sharing of electron pairs between atoms, promoting molecular stability and integrity. In this case, the two oxygen atoms engage in such a bond to form the stable molecular structure of O2.
However, the conversation does not end with the acknowledgment of O2 as a molecule. When proceeding to classify it as a compound, one must address the criterion of diversity in elemental composition. The essential distinction lies in the notion that compounds must incorporate at least two different types of atoms. Hence, while O2 embodies the properties of a molecule, it does not fulfill the requirements to be classified as a compound.
This leads to a broader inquiry regarding the nuances of elemental molecules. The category includes not only O2 but also other diatomic molecules such as N2 (nitrogen), H2 (hydrogen), and Cl2 (chlorine). Each of these substances is likewise categorized solely as molecules due to their composition of two identical atoms. Therefore, they dance along the border of molecularity and elemental status but remain firmly within the sphere of elemental molecules rather than compounds.
In unpacking further complexities, the distinction of O2 prompts additional questions. For instance, how does this classification impact our understanding of chemical reactions and interactions in various environments? The role of O2 within biology and Earth’s atmosphere accentuates its significance; it is vital for respiration in aerobic organisms and participates crucially in combustion reactions. It stands as a vital mediator of energy transformations, thus shaping the very fabric of life on Earth.
To illustrate, when O2 engages in reactions, such as combustion, it reacts with compounds, rather than with other identical atoms. These reactions underscore the essential differentiation between molecules and compounds. During a combustion event, for instance, O2 interacts with carbon compounds, breaking down their structure while facilitating the release of energy. This capacity to act upon differing substances exemplifies the critical role O2 plays, although it remains a molecule—in essence, a simple assemblage lacking the diverse elemental characteristics that define compounds.
In contemplating the broader chemical landscape, the discussion of O2 pivots to molecular interactions and the nature of elemental existences. This conversation introduces educational opportunities regarding molecular geometry, reactivity, and valency—the very cornerstones of chemical understanding. While O2 stands solely as a molecule, the implications of its reactivity propel chemists towards multifaceted explorations within the domain of molecular and compound chemistry.
Moreover, the interaction of O2 with various other elements gives rise to an array of compounds, including metal oxides and organic reagents. Each interaction serves as a reminder of the overarching significance that elemental molecules possess within comprehensive chemical systems. While O2 may reside on the molecule-laden shores, the prominent compounds that emerge within reactions demonstrate the expansive narrative of chemistry. The dichotomy, therefore, not only enriches our vocabulary but also enlivens our scientific pursuits.
To summarize, O2 is unequivocally categorized as a molecule. However, due to its homogenous atomic structure, it does not qualify as a compound. This distinction provides fertile ground for further inquiry and learning, as it reflects the broader principles underlying chemistry. Recognizing these contrasts offers insights into the nature of substances, enriching our understanding while simultaneously fostering the curiosity that drives scientific discovery.
In the annals of chemistry, O2 stands as a prime example of how simple atomic structures can lead to profound impacts on life and nature. Such realizations serve to invigorate discussions surrounding molecules and compounds, pushing the boundaries of knowledge—proving that even a singular question can unravel the intricacies of a vast chemical world.