Matter is the cornerstone of all physical existence, enveloping the universe across a myriad of conditions and forms. It is defined as anything that possesses mass and occupies space. Matter comprises particles that exhibit both wave-like and particle-like properties, an intriguing characteristic that delves into the realms of quantum mechanics. Understanding matter involves an exploration of its composition, structure, interactions, and the fundamental states it inhabits.
At its most fundamental level, matter can be broken down into elements, the basic building blocks found on the periodic table. Elements consist of atoms, which are the smallest units retaining the properties of the element. Atoms themselves are composed of subatomic particles: protons, neutrons, and electrons. Protons and neutrons form the nucleus at the atom’s center, while electrons orbit this nucleus in defined energy levels or shells. This atomic structure sets the stage for the interactions and transformations that occur in different states of matter.
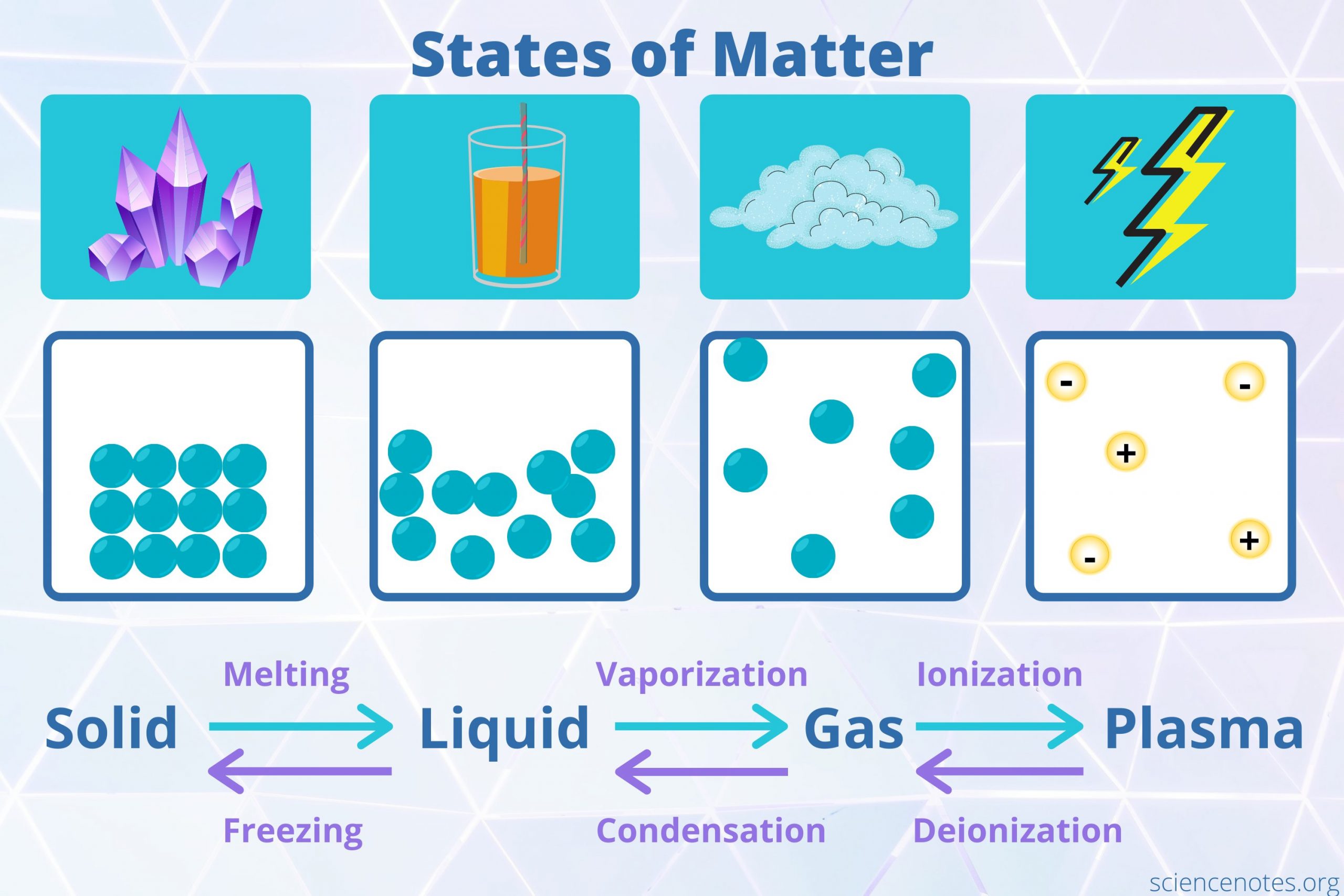
The classical framework posits that matter exists in four primary states: solid, liquid, gas, and plasma. Each state is characterized by distinct properties, intermolecular forces, and energy levels, resulting in different behaviors and appearances.
1. Solid State
In solids, particles are closely packed together, often in a regular arrangement known as a crystal lattice. The strong intermolecular forces that bind these particles together confer structure and rigidity. Solids have a definite shape and volume, as they cannot easily change their form unless subjected to substantial force or heat. The energy in solids is the lowest compared to the other states, typically freezing the particles in fixed positions. Various solid materials demonstrate diverse characteristics—metals exhibit malleability and ductility, while non-metals may be brittle.
2. Liquid State
Moving on to liquids, the arrangement of particles shifts; they are in close proximity but not in fixed positions, allowing for fluid movement. Liquids maintain a definite volume but take the shape of their container. The intermolecular forces in liquids are less formidable than in solids, which grants them the ability to flow. This state is further characterized by cohesion (the attraction between similar molecules) and adhesion (the attraction between different molecules), influencing phenomena such as surface tension and capillary action.
3. Gaseous State
Gases are marked by their particles being far apart, with negligible intermolecular forces at play. This state occupies volume and shape dictated by the container, exhibiting a propensity to expand indefinitely. The kinetic energy of gas particles is significantly higher than in solids and liquids, engendering rapid movement and frequent collisions. This dynamic nature explains why gases can disperse and fill available space, as outlined by the ideal gas laws. Moreover, gases can undergo invariant transformations; increasing temperature typically escalates pressure, while decreasing volume can elevate temperature, as described by Gay-Lussac’s Law.
4. Plasma State
Beyond the traditional states of matter lies plasma—a highly ionized gas composed of free electrons and ions. Plasmas are prevalent in the universe, notably within stars, including our sun. The thermal energy in plasmas is remarkably high, facilitating the detachment of electrons from their atomic nuclei, resulting in a collection of charged particles. Due to their collective behavior and electromagnetic properties, plasmas respond to magnetic fields, manifesting in unique structures such as solar flares and auroras. They are also harnessed in various technologies, such as fluorescent lights and plasma TVs.
Transition Between States
Transitions between these states of matter are governed by changes in energy, typically influenced by temperature and pressure. Phase transitions occur when energy is added or removed from a material. For instance, heating a solid can induce melting, transforming it into a liquid. Conversely, cooling a gas can lead to condensation, precipitating a liquid state. The study of these transitions reveals essential thermodynamic principles and the underlying order amid apparent chaos.
Other States of Matter
Beyond the four classical states, researchers have identified other states of matter under extreme conditions. These include:
- Bose-Einstein Condensate (BEC): Formed at temperatures approaching absolute zero, resulting in a state where particles occupy the same quantum state, behaving as a single quantum entity.
- Fermionic Condensate: Similar to BEC, but formed from fermions instead of bosons, exhibiting superfluid characteristics at low temperatures.
- Quantum Spin Liquid: A state of matter characterized by entangled quantum states, resulting in a disordered arrangement devoid of long-range magnetic order even at absolute zero.
- Supersolid: A phase that exhibits properties of both solids and superfluids, allowing protons to flow without friction.
Conclusion
In conclusion, matter is an intricate tapestry woven from fundamental particles manifesting in various states. The classical representation of solid, liquid, gas, and plasma provides a framework for understanding the diversity of materials in the universe. As scientific inquiries delve deeper, new states of matter are being uncovered, expanding our comprehension of the physical world and challenging existing paradigms. Continuous exploration of matter and its transitions offers profound insights into the fundamental nature of the universe and the intricate forces that govern our reality.