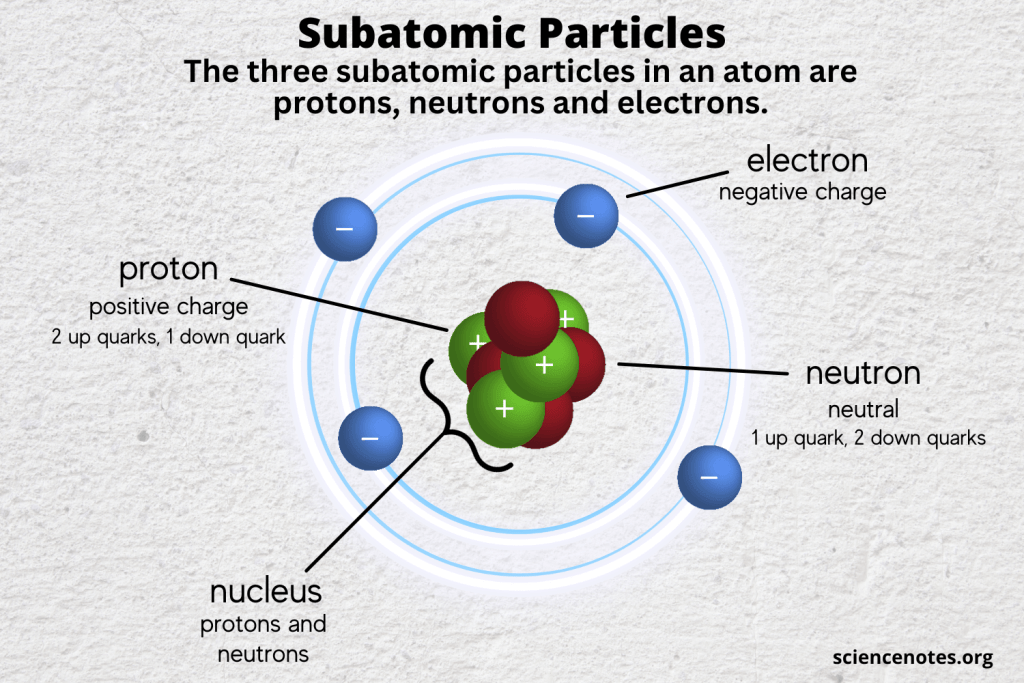
The structure of an atom is a marvel of natural organization, akin to a solar system where the planetary bodies circle a central sun. Yet, within this intricate cosmic dance lies a distinction among its myriad constituents, primarily centered around the concept of mass. In this exploration, we delve into the realm of atomic particles, discerning which among them are classified as heavy particles. To pave the way for understanding, it is essential to introduce the players in this atomic tableau: protons, neutrons, and electrons, with the focus resting squarely on the former two.
Atoms are composed of a nucleus surrounded by electrons, creating a miniature cosmic order. Within the nucleus resides the heavyweight champions of the atomic world: protons and neutrons, collectively known as nucleons. Their mass is substantially greater than that of electrons—the renegade particles that waltz around the atom’s periphery.
Protons: The Charge Bearers
Protons are positively charged particles that inhabit the nucleus of an atom. Each proton possesses a mass of approximately 1.67 x 10-27 kilograms, contributing significantly to the overall mass of the atom. They act as the gravitational core, demonstrating a propensity to attract electrons and maintain atomic integrity. The number of protons found in an atom dictates its elemental identity, forming the basis of the periodic table. For example, hydrogen boasts a single proton, while uranium possesses a staggering 92. This relationship between protons and element identity offers a gateway into the world of chemistry and beyond, as the interactions initiated by protons lay the foundation for molecular structures.
The significance of protons extends beyond their mere presence in the nucleus. These particles partake in various nuclear interactions that can alter the stability of an atom. The balance between the number of protons and neutrons determines whether an atom is stable or prone to radioactive decay. Hence, the proton not only serves as the hallmark of an element but also influences the atom’s behavior across various external conditions.
Neutrons: The Silent Guardians
In the shadow of protons reside neutrons, the indifferent counterparts that contribute emblematically to the atomic nucleus. With a mass nearly equivalent to that of protons, neutrons exhibit no electric charge, rendering them neutral entities in the nucleus. This neutrality, however, belies their critical role in conferring stability to the nucleus. For many elements, the ratio of neutrons to protons is crucial; a delicate equilibrium that ensures the forces holding the nucleus together—primarily the strong nuclear force—are sufficient to counteract the electromagnetic repulsion between positively charged protons.
Neutrons, while heavy particles in their own right, represent a paradox in atomic structure. Variants of a particular element known as isotopes arise from differing numbers of neutrons. For instance, carbon has both carbon-12 and carbon-14 isotopes, differing by their neutronic count. The presence of these isotopes accentuates the complexity of atomic behavior, particularly in the context of nuclear reactions and radioactive decay, which are ever-present in various fields of science, including medicine, archaeology, and energy production.
The Lightweight Dancers: Electrons
Contrasting starkly with protons and neutrons, electrons are the fleet-footed entities whirring about the nucleus. Possessing a mass 1/1836 that of a proton, electrons are often overlooked in discussions of atomic weight. Their negligible mass does not diminish their importance, as they are the linchpin of chemical reactions and bonding. The configurations of electrons in their shells dictate the reactivity of an atom, exemplifying the atom’s behavior in interaction with other substances in myriad chemical contexts. While not heavy particles, electrons provide a dynamic counterbalance to the static heaviness of protons and neutrons, illustrating the intricate dance of forces at play.
Mass vs. Stability
In the spectrum of atomic particles, the distinction between mass and stability is paramount. Heavy particles, protons, and neutrons forge the backbone of atomic structure, their mass a fundamental characteristic that sets the stage for the universe’s broader fabric. Stability arises not merely from the mass of nucleons but also from their intricate interplay. An atom teeters on the precipice of radioactivity or stability largely due to the meticulous balancing act carried out by protons and neutrons.
Conclusion: The Heft of Atomic Composition
To summarize, the heavy particles within an atom—namely protons and neutrons—occupy a privileged position in the hierarchical structure of matter. These nucleons are not only integral to defining the mass of an atom, but they also orchestrate the stability and reactivity that governs chemical interactions. Understanding the roles of protons and neutrons allows for greater insights into the atomic foundation of our world, illuminating the paths of both stability and transformation that atoms can undertake. Through the harmonious interplay of heavy particles and their light counterparts, the realm of atomic physics continues its endless dance, a testament to the complexity and elegance of our universe.