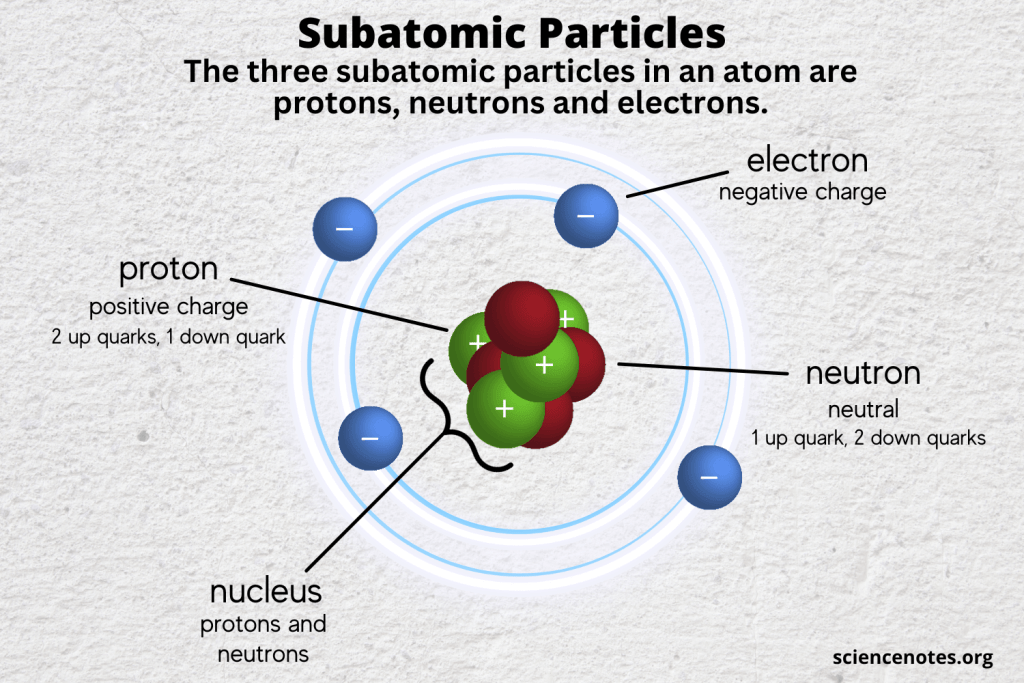
Atoms, the fundamental building blocks of matter, are defined by their subatomic constituents: protons, neutrons, and electrons. Understanding the stability of an atom necessitates a thorough examination of these particles and their interactions. Stability influences an atom’s ability to exist without undergoing spontaneous decay, which can significantly alter its chemical properties and interactions with other atoms. This exploration will delve into the roles played by the three primary subatomic particles and the forces that hold them together.
1. Protons: The Nucleus’s Positive Charge
Protons are positively charged subatomic particles found in the nucleus of an atom. The number of protons, known as the atomic number, determines the identity of the element and plays a pivotal role in its chemical behavior. For example, an atom with one proton is hydrogen, while one with six protons is carbon. An essential aspect of protons is their interaction through the electromagnetic force.
Despite their positive charge, protons are housed together within the nucleus, where repulsive electromagnetic forces should ostensibly force them apart. However, the stability provided by nuclear forces—specifically the strong nuclear force—compensates for this repulsion. This fundamental force, which operates at subatomic distances, binds protons (and neutrons) together, thus maintaining the integrity of the atomic nucleus.
Nevertheless, an imbalance in the proton-to-neutron ratio can lead to instability. For instance, heavier elements often possess more neutrons than protons, a phenomenon that can enhance nuclear stability. Yet, when these ratios become too disproportionate, the nucleus becomes susceptible to decay, leading to various forms of radioactive emissions, such as alpha or beta decay.
2. Neutrons: The Stabilizing Force
Neutrons are electrically neutral particles that coexist with protons in the atomic nucleus. Their primary role extends beyond simply adding mass; neutrons are crucial for mitigating the repulsive forces among protons. By providing an additional strong nuclear force without contributing to electromagnetic repulsion, neutrons effectively enhance nuclear stability.
The presence of neutrons influences the binding energy of the nucleus, the energy required to disassemble the nucleus into its constituent particles. An ideal ratio of neutrons to protons is vital for preventing nuclear instability, which is characterized by an excess or deficiency of neutrons. Isotopes of an element, which differ in neutron count, exhibit varying degrees of stability. Stable isotopes, such as carbon-12 (six protons and six neutrons), contrast sharply with radioisotopes like carbon-14, which undergoes beta decay due to its higher neutron-to-proton ratio.
In summary, the neutron’s fundamental role in atomic structure is underscored by its critical function in bolstering nuclear stability; too few or too many neutrons can tip the balance toward instability, making it imperative for various atoms to maintain their neutron count within a specific range relative to their protons.
3. Electrons: The Cloud of Negativity
Electrons, negatively charged particles residing in electron shells surrounding the nucleus, play an essential role in an atom’s chemical properties and interactions. While they do not significantly contribute to an atom’s mass, their arrangement dictates how an atom interacts with other atoms through covalent and ionic bonding.
The stability of an atom is subtly affected by the behavior of its electrons, primarily through their distribution and energy levels. Electrons occupy quantized energy levels, forming what is referred to as the electron cloud. An atom is most stable when its outermost electron shell is filled, a concept known as the octet rule. Atoms tend to seek stability by gaining, losing, or sharing electrons, leading to the formation of chemical bonds.
For instance, in ionic compounds, atoms transfer electrons to achieve full outer shells, yielding charged ions that are held together by strong electrostatic forces. In covalent compounds, atoms share electrons to attain stability. Thus, an imbalance in electrons—either through added or removed electrons—leads to ionization, which can provoke reactions that jeopardize the stability of the atom.
4. The Forces At Play: Strong Nuclear Force and Electromagnetic Force
The stability of an atom is fundamentally governed by the interplay of two primary forces: the strong nuclear force and the electromagnetic force. The strong nuclear force is a short-range force that acts between protons and neutrons, holding the nucleus intact against the dissociative tendencies of electromagnetic repulsion among like-charged protons. Its effectiveness diminishes with distance, which is why it is crucial for the particles to be in close proximity within the nucleus.
Conversely, the electromagnetic force operates over longer distances and is responsible for the interactions between electrons and the positively charged nucleus. The intricate balance between these forces determines the overall stability of the atom. A stable atom maintains equilibrium, where the attractive forces of the strong nuclear force effectively counteract the repulsive forces among protons while electrons occupy stable energy configurations, thus avoiding excessive repulsion among them.
Conclusion: The Interdependence of Subatomic Particles
The stability of an atom emerges from the complex and interdependent relationships among its subatomic particles. Protons define the identity and charge of the atom, neutrons stabilize the nucleus, and electrons govern the atom’s reactivity and chemical behavior. A delicate equilibrium among these forces and particles determines whether an atom will remain stable or succumb to various forms of decay. Through an understanding of these interactions, we can gain deeper insights into the fundamental nature of matter and the processes that underpin chemical stability in the universe.