The universe, with its intricate tapestry of matter, unfolds in a grand play of existence—where atoms dance, elements weave, and molecules collaborate. Defining these fundamental units of matter not only enriches our understanding of chemistry but also lays the groundwork for diverse scientific disciplines, from biochemistry to material science. Hence, elucidating the difference between atoms, molecules, and elements becomes paramount in the quest for knowledge.
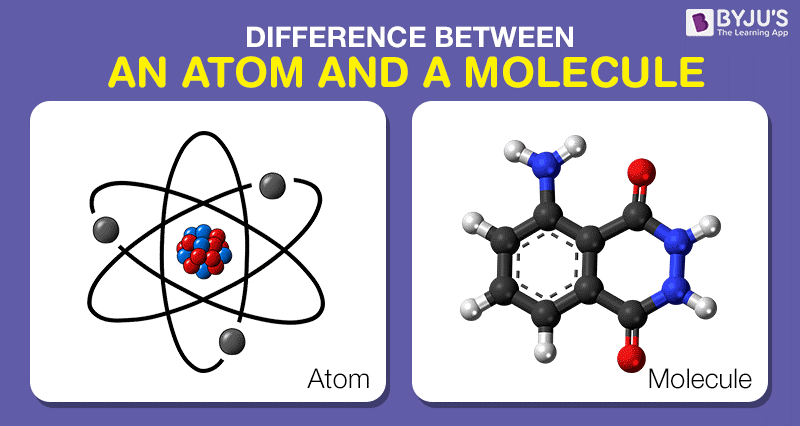
At the foundational level of matter lies the atom, often regarded as the quintessential building block of all substances. An atom can be likened to an indelible imprint on the fabric of the universe; imperceptibly small yet overwhelmingly significant. Composed of a nucleus—a central hub of protons and neutrons—surrounded by a cloud of electrons, atoms encapsulate the essence of an element. Each element, characterized by its unique number of protons—also known as the atomic number—distinguishes itself in the periodic tapestry. For instance, a single hydrogen atom, with its solitary proton, carries the identity of hydrogen, while a mammoth uranium atom boasts an imposing 92 protons.
Moving from the solitary atom to the collective narrative of elements, one discerns a qualitative leap in complexity. An element represents a pure substance that cannot be decomposed into simpler substances through chemical reactions. Elements are akin to the diverse colors of paint on an artist’s palette—each possessing distinctive properties. When we talk about elements like oxygen, carbon, or gold, we refer to the purest forms of matter that retain their identity through myriad reactions. This purity is what enables elements to combine with one another under specific conditions to create compounds, bridging the chasm between basic and advanced chemistry.
Yet, this is but one chapter in the expansive story of matter. When elements intermingle, they sometimes unite to form molecules, setting the stage for a more intricate saga. A molecule can be defined as a group of two or more atoms bonded together by covalent bonds. This intricate web of relationships allows molecules to manifest as the very substances that breathe life into the world around us. For instance, a water molecule (H₂O), composed of two hydrogen atoms and one oxygen atom, exemplifies how simple atomic constituents can unite to give rise to a wholly new entity with distinct properties, capable of supporting life.
But the distinctions and interactions between atoms, elements, and molecules extend beyond mere definitions. They evoke a metaphorical narrative, where atoms are the individual notes in a symphony, elements are the distinct melodies, and molecules are the harmonies that emerge when these melodies intertwine. Each component plays its role—the atom provides the essence, the element offers the identity, while the molecule displays the output of combined efforts.
Diving deeper into this analogy, we find that atoms can be classified further into elemental atoms and molecular atoms. The former exists as standalone entities, whilst the latter participates in the formation of compounds. Consider the noble gases—like helium and neon—which exist as solitary atoms in nature, showcasing the stability of their atomic structure. In contrast, diatomic molecules such as nitrogen (N₂) and oxygen (O₂) emphasize the necessity of bonding in nature, fundamentally altering their properties in the process.
Furthermore, let us not overlook the crucial distinction between macroscopic and microscopic perspectives when contemplating these fundamental units of matter. On a macroscopic scale, elements and compounds are discernible and quantifiable—each presenting unique physical properties such as boiling points, melting points, and solubility. Take sodium chloride, or table salt, for instance; it stands as a compound composed of sodium and chlorine atoms—demonstrating a conspicuous divergence in properties when compared to its constituent elements. In the microscopic realm, however, the interplay becomes far more complex, showcasing the quantum behavior of atoms and molecules, where principles such as wave-particle duality and electron shell configurations come into play.
The study of these differences extends into other realms of scientific inquiry, particularly in the fields of chemistry and physics. The chemical bonds that form between atoms to create molecules give rise to diverse interactions and reactions that form the basis of organic and inorganic chemistry. These bonds can be covalent, ionic, or metallic, each governing the behavior and properties of the resulting substances. Understanding these bonding mechanisms unveils pathways to innovative materials and pharmaceuticals, reshaping our technological landscape.
Moreover, from a philosophical standpoint, atoms, elements, and molecules beckon profound inquiries into the nature of existence itself. What are the implications of such distinctions on our understanding of life, substance, and reality? If atoms are our smallest units of matter, what does their behavior reveal about the fundamental fabric of the universe? The science of matter transcends beyond identity; it invokes the interconnectedness of all things, where the simple merges into the complex, forming the diverse tapestry that is reality.
In conclusion, the differences between atoms, elements, and molecules encapsulate a narrative of complexity and wonder. These fundamental units serve not only as the scaffolding of matter but also as gateways to understanding the intricacies of science and existence. Atoms function as the basic building blocks, elements present their pure identities, and molecules reveal the melodious interplay of atoms and bonds. This triad forms the cornerstone of material reality, inviting us to explore deeper into the realms of chemistry, physics, and beyond. With each revelation, we are reminded of the profound elegance underlying the seemingly mundane world around us.