Protein folding is a quintessential process essential to the functionality of biological macromolecules. This intricate mechanism not only determines the three-dimensional conformation of proteins but also dictates their activity in metabolic pathways and cellular functions. The discourse surrounding protein folding often oscillates between the domains of biophysics and biochemistry. This article elucidates the nuances of protein folding, exploring its implications within both disciplines, while positing that it is, indeed, a confluence of biophysics and biochemistry.
To initiate this exploration, it is imperative to understand the fundamental aspects of protein folding. Proteins are polypeptides composed of amino acids which are inherently linear sequences. However, upon synthesis, these linear chains undergo stochastic processes that lead to the emergence of well-defined, three-dimensional structures. This transformation is governed by the intrinsic physicochemical properties of the amino acids, where hydrophobic interactions, hydrogen bonding, ionic interactions, and van der Waals forces play pivotal roles.
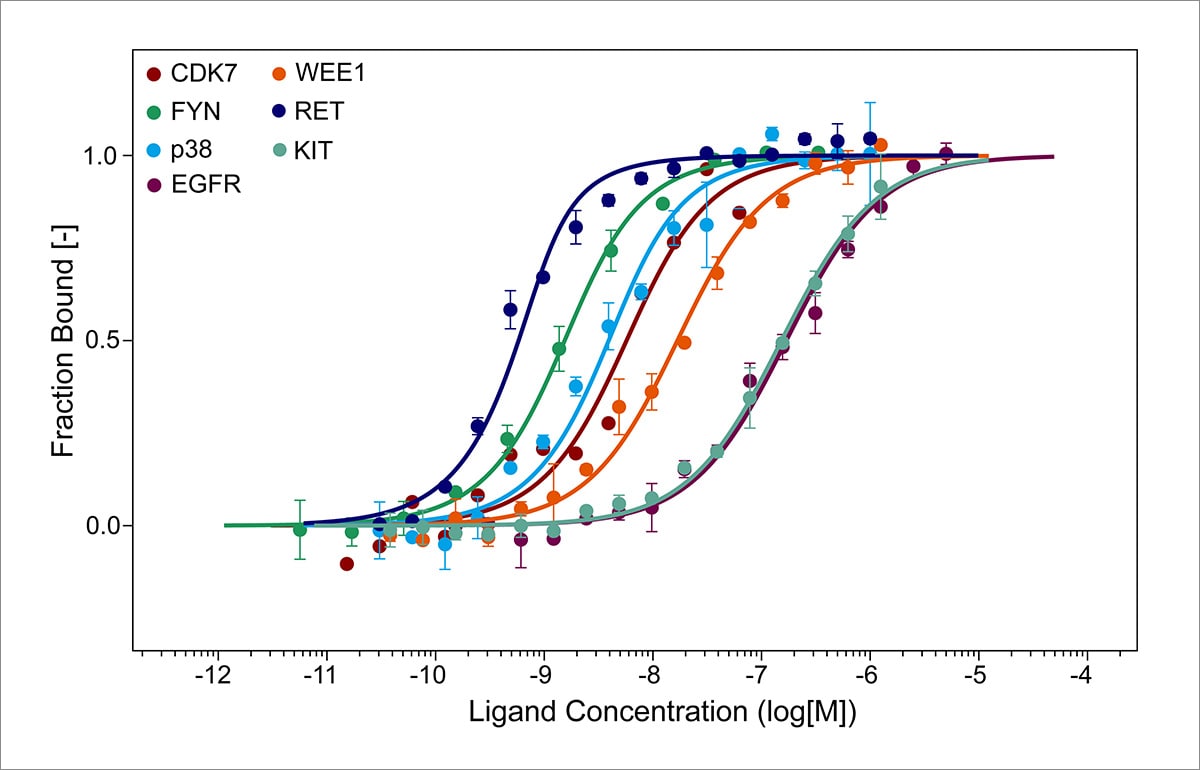
Biophysics enters the arena with its focus on the physical principles governing these interactions. The folding process can be depicted as a multidimensional energy landscape, introducing concepts such as free energy minima and protein stability. Techniques such as X-ray crystallography, nuclear magnetic resonance (NMR), and cryo-electron microscopy allow for the visualization of protein structures at various folding states. Biophysicists utilize computational modeling and simulations, employing methods such as molecular dynamics, to elucidate the dynamics involved in folding pathways. This approach emphasizes the importance of energy minimization and kinetic barriers characteristic of the folding process.
However, while the physical interactions are critical, the biochemical context cannot be overlooked. Enter biochemistry, which delves into the energetic and kinetic parameters driving protein folding. The role of chaperones—a class of proteins that assist in the proper folding of other proteins—exemplifies biochemical intervention in the folding mechanism. Chaperonins like GroEL/GroES illustrate how cellular conditions modulate the folding pathways, steering substrates towards their native conformations and preventing misfolding, which can lead to aggregation and various pathologies.
Moreover, the biochemical environment, characterized by factors such as pH, temperature, and ionic strength, profoundly influences protein folding. Enzymes involved in post-translational modifications—such as glycosylation or phosphorylation—can significantly alter a protein’s stability and folding kinetics. These modifications effectively bridge the biochemical with the biophysical, as the structural conformation post-modification may exhibit altered physicochemical properties compared to its unmodified counterpart.
A deeper dive into models of protein folding reveals a dichotomy in perspectives. The hierarchical model of protein folding suggests that proteins fold through intermediate states, conforming to a funnel-like energy landscape. Conversely, the nucleation-condensation model posits that specific regions of the polypeptide sequence, known as nucleation sites, initiate folding, with subsequent condensation of non-local interactions guiding the folding process towards its stable state. Both models highlight the interplay of biophysical elements, such as the influence of kinetic traps and thermodynamic stability, and underscore the biochemical intricacies, emphasizing the role of catalytic agents and environmental influences on the folding narrative.
Protein misfolding diseases further underscore the intersection of biophysics and biochemistry. Conditions such as Alzheimer’s, Parkinson’s, and prion diseases are a result of aberrant folding pathways that culminate in the aggregation of proteins, leading to toxic species within cells. Understanding these processes necessitates a dual-lens approach: biophysical techniques provide insights into the structural aberrancies while biochemical analysis elucidates the metabolic perturbations accompanying these fold-related pathologies. Here, the importance of collaboration between biophysicists and biochemists is underlying, as it grants a more holistic understanding of the myriad factors influencing the proper folding of proteins.
Consequently, education and research within the realm of protein folding are increasingly adopting an interdisciplinary methodology. Advanced programs now emphasize the convergence of biophysics and biochemistry, fostering an integrated comprehension among students and researchers alike. As the significance of systems biology continues to burgeon, the exploration of protein interactions will predominantly benefit from this multidisciplinary approach.
In summary, while protein folding has traditionally been dissected through specific disciplinary lenses, it is irrefutably a phenomenon that thrives on the confluence of biophysics and biochemistry. The complexity and sophistication of protein folding underscore the necessity for holistic and collaborative research approaches that encompass both domains. As we strive to unravel the labyrinth of protein folding, recognizing that it embodies the principles of both physical and biological sciences is paramount. Such acknowledgment is not merely academic but could harbor profound implications for therapeutic strategies aimed at combating misfolding-associated diseases, thus enriching our understanding of life at the molecular level.