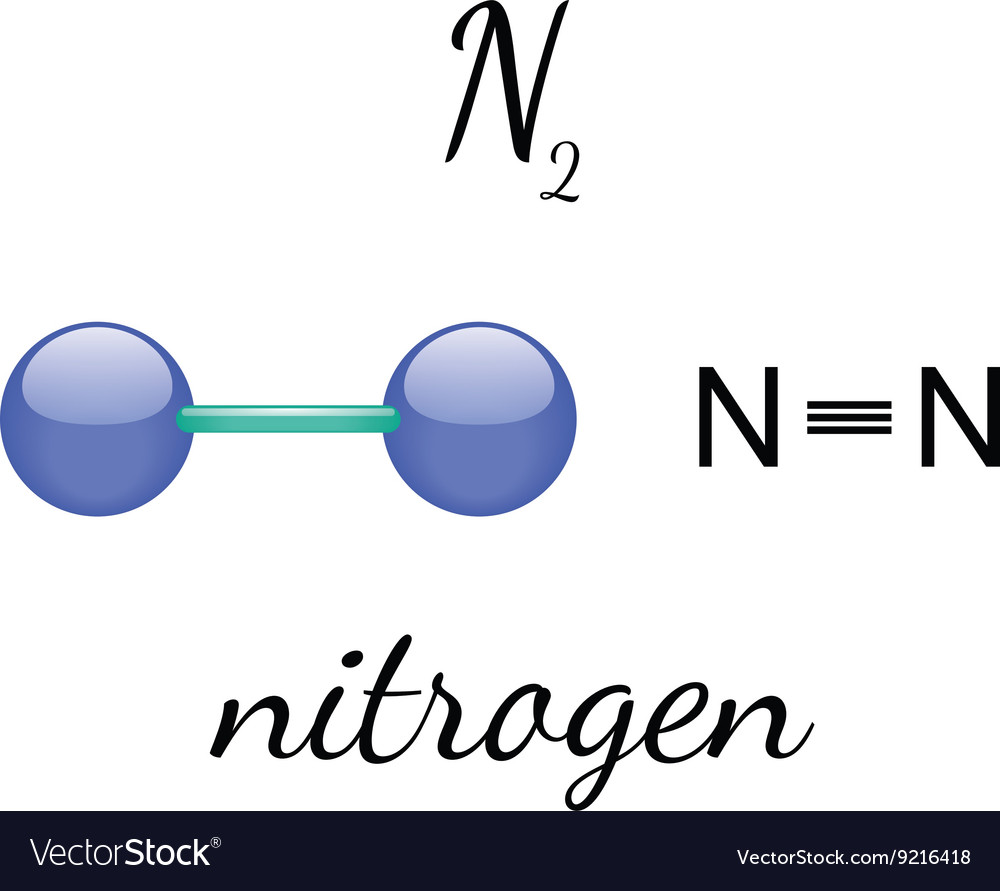
In the fascinating realm of chemistry, the distinctions between atoms and molecules serve as foundational concepts. Atoms, the quintessential building blocks of matter, are indivisible entities characterized by their specific arrangement of protons, neutrons, and electrons. Molecules, in contrast, emerge when two or more atoms unite, culminating in a more complex structure that reflects the concert of chemical bonds. This distinction leads us to the inquiry regarding nitrogen, specifically N2. Is N2 an atom or a molecule? Herein lies an opportunity to explore nitrogen’s dualistic nature, intricacies of its molecular formation, and its pivotal role in both nature and industry.
To understand N2, one must first delve into the architectural framework of the nitrogen atom. Nitrogen, located in group 15 of the periodic table, is an element enriched with five valence electrons. This unique electron configuration sets the stage for the atom’s proclivity to form strong covalent bonds. The N2 molecule is a diatomic molecule, meaning it consists of two nitrogen atoms bonded together. This bond is forged through what is known as a triple bond, characterized by the sharing of three pairs of electrons. Here lies the beauty of diatomic nitrogen; it showcases the elegance of atomic connectivity and chemical interaction.
Visualize the nitrogen atoms entwined, much like dancers in a synchronized ballet. Their shared electrons act as a tether, permitting the particles to retain their individual identities while simultaneously forming a cohesive whole. The formation of the N2 molecule is a striking illustration of the statement that unity can yield greater strength. Furthermore, this triple bond, one of the strongest in chemistry, underscores the resilience and stability of the N2 molecule in various environments.
Yet, it is essential to clarify that N2, despite being constituted of individual nitrogen atoms, unequivocally qualifies as a molecule. The American Chemical Society defines a molecule as the smallest particle of a substance that retains its chemical properties. Therefore, N2, being comprised of two nitrogen atoms joined by a robust triple bond, meets this definition with aplomb.
Diving deeper into the enigmatic properties of N2, one discovers its unique appeal in the natural world. Nitrogen constitutes approximately 78% of Earth’s atmosphere, acting as an inert filler in the gaseous expanse that envelops our planet. Its inertness arises from the strong triple bond that renders N2 remarkably stable. This stability is a double-edged sword; while it ensures N2‘s persistence in the atmosphere, it also necessitates specific conditions for its reactivity.
As the phenomenon of photosynthesis unfolds, we witness N2 in a complex interplay with other elements. Although nitrogen in its diatomic state remains unreactive, it is through natural processes such as nitrogen fixation that this molecule is converted into reactive compounds. These compounds are essential for the synthesis of amino acids, the building blocks of proteins, ultimately sustaining life on the planet. Here, we observe a metamorphosis—the conversion of a simple molecule into a vital element of complex biochemical frameworks.
From the biosphere, the journey of nitrogen leads us into the realms of industry. N2 gas serves not only as an inert atmosphere in various manufacturing processes but also plays a crucial role in the production of fertilizers, explosives, and pharmaceuticals. The Haber-Bosch process exemplifies our ability to industrially synthesize ammonia from nitrogen and hydrogen gases, displaying the transformative power of chemistry when it comes to ensuring food security and facilitating industrial advancement. Thus, N2 transcends its identity as a mere atmospheric component; it becomes a linchpin in the tapestry of human innovation.
The fascination with N2 extends beyond its chemical properties; it also encapsulates a philosophical reflection on the nature of existence. The stability and inertness of N2 can be viewed as a metaphor for the equilibrium found in our own lives—a reminder that not all elements of existence need to be reactive or dynamic. Sometimes, the presence of inertness fosters balance, allowing for the complex interactions of growth and development to unfold in their own time.
In summation, N2 is undoubtedly a molecule, forged from the union of two nitrogen atoms bound by a tenacious triple bond. Yet, its implications reach far beyond the laboratory, influencing ecosystems, industrial practices, and philosophical musings. To consider N2 solely as a diatomic molecule is to overlook its potential as a cornerstone of life and a participant in humanity’s narrative of progress. Therefore, N2 stands not just as a molecule amidst countless others in the universe, but as a symbol of connectivity, resilience, and transformation—elements intrinsic to both the scientific realm and the continuum of existence itself.