Hydrocarbons, the cornerstone of organic chemistry, are often recast in discussions of materials science, energy production, and biological processes. Their fundamental structure and varied forms yield captivating insights into the nature of matter. This article elucidates the query: Is a hydrocarbon an atom or a molecule? To answer this, a nuanced exploration of chemical composition, molecular bonding, and the broader implications of hydrocarbons in scientific inquiry is warranted.
Firstly, it is imperative to delineate the terms involved in this inquiry. An atom, the basic unit of matter, comprises a nucleus formed from protons and neutrons, surrounded by a cloud of electrons. In contrast, a molecule represents a more complex structure, a culmination of two or more atoms bonded together. Thus, positing hydrocarbons as mere atoms would be a significant mischaracterization. Hydrocarbons are inherently molecular entities, epitomizing compounds that consist exclusively of hydrogen and carbon atoms.
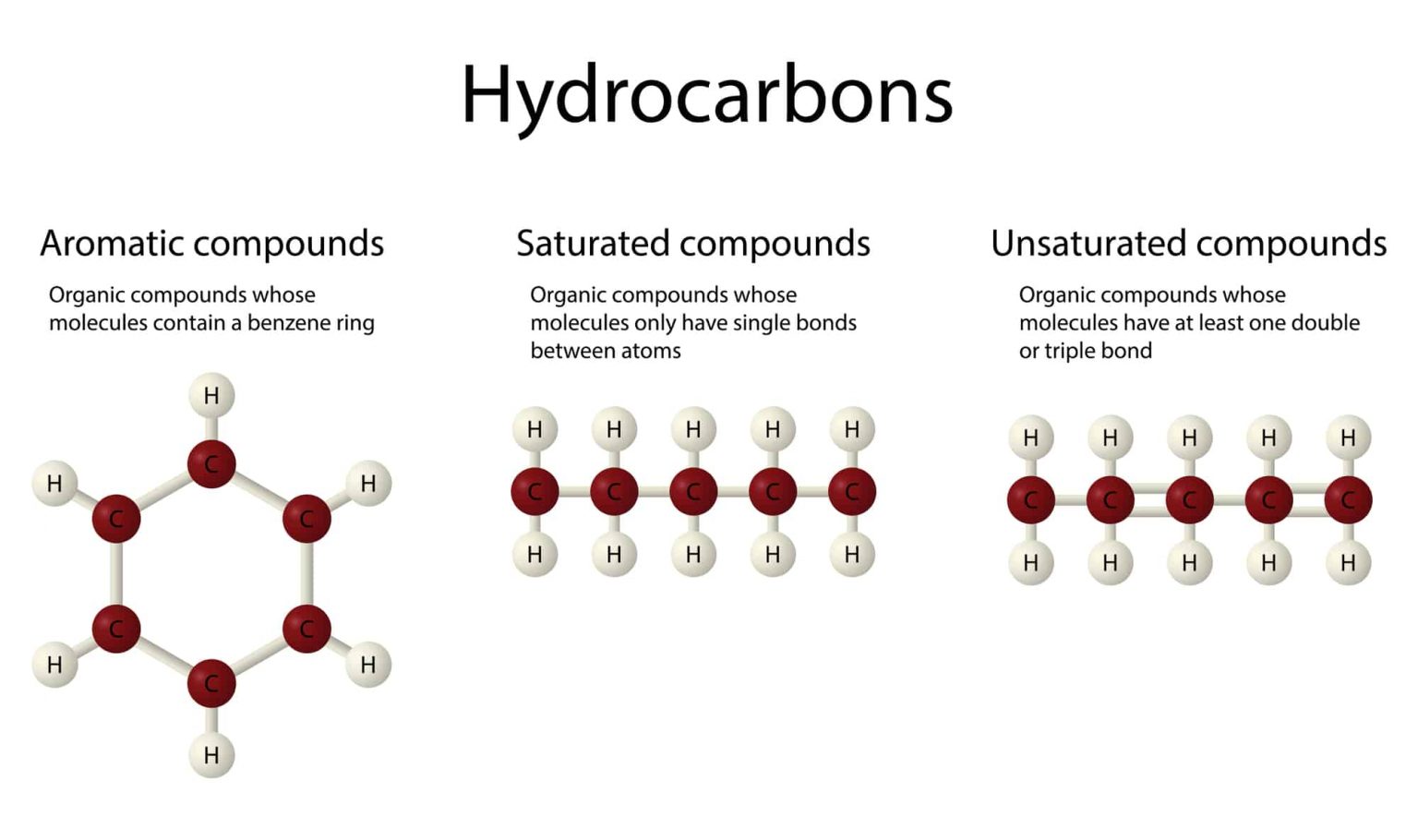
At its simplest level, a hydrocarbon can be classified as either aliphatic or aromatic. Aliphatic hydrocarbons, including alkanes, alkenes, and alkynes, present a linear or branched structure that forms chains, whereas aromatic hydrocarbons are characterized by one or more phenolic rings, exhibiting delocalized pi electrons across the ring structure. This distinction is not merely trivial; it introduces the concept of geometry and bonding angles, playing a critical role in the reactivity and properties of these molecules.
The significance of hydrocarbons extends beyond their classification. The carbon atom, with its tetravalent nature, demonstrates an unparalleled ability to form stable covalent bonds with hydrogen and other elements. This property facilitates the emergence of a vast array of hydrocarbon molecules, ranging from simple methane (CH4) to complex polycyclic aromatic hydrocarbons. The prevalence of carbon-based life, its central role in biological macromolecules, and hydrocarbons’ ubiquitous presence in fossil fuels underscore the relevance of these structures in the cosmic tapestry.
Examining the foundational building blocks of hydrocarbons reveals staggering diversity. The simplest hydrocarbons—methane, ethane, and propane—serve as primary examples, each offering unique properties dictated by the number of carbon atoms present. Methane, a singular carbon atom surrounded by four hydrogen atoms, epitomizes aliphatic simplicity and is pivotal in energy generation as a natural gas component. As we ascend the molecular ladder, the formation of long, branched chains creates substances with entirely different physical and chemical characteristics. This fossil heritage not only fuels combustion engines, but it is integral to the manufacture of countless chemical products, ranging from plastics to pharmaceuticals.
Furthermore, variations in hydrogens’ saturation levels yield different hydrocarbons: saturated hydrocarbons (alkanes) contain only single bonds between carbon atoms, while unsaturated hydrocarbons (alkenes and alkynes) exhibit double and triple bonds, respectively. This saturation variance underpins the reactivity of hydrocarbons, guiding transformations in chemical synthesis that are essential in industrial applications and laboratory settings. Understanding these structural intricacies equips chemists and physicists with the knowledge requisite for manipulating bond formation and breaking in hydrocarbon molecules.
The environmental ramifications of hydrocarbons further complicate their status in contemporary discourse. The combustion of hydrocarbon fuels for energy releases carbon dioxide, a notorious greenhouse gas implicated in climate change. This creates a dichotomy within the scientific community: while hydrocarbons are invaluable for their energy potential, reliance on fossil fuel resources poses profound challenges to sustainability. The pursuit of alternative energy sources, such as biofuels and renewable energies, emerges as a response to an increasingly urgent ecological crisis. These developments elucidate the intricate linkage between hydrocarbon consumption and global climate phenomena.
The quest for understanding hydrocarbons also extends into the realms of nanotechnology and materials science. Advances in synthesizing hydrocarbon derivatives have led to innovations in creating nanomaterials with extraordinary properties, bolstered by the unique attributes of carbon itself. Graphene and carbon nanotubes, derived from the manipulation of hydrocarbon structures, signify a frontier where molecular chemistry interfaces with physical applications, heralding a new era in engineering, electronics, and medicine.
Additionally, hydrocarbons exhibit fascinating interactions at a molecular level, leading to intriguing phenomena, such as solubility and hydrophobicity. The disparate affinities between hydrocarbons and polar solvents reveal essential principles in separation techniques, frequently employed in both organic and analytical chemistry. Understanding solubility governs reactions and is essential in pharmacokinetics, where the absorption and distribution of drugs hinge upon molecular interactions akin to those exhibited by hydrocarbons.
Conclusively, hydrocarbons unequivocally manifest as molecules, rather than atoms, composed of carbon and hydrogen in various configurations. Their molecular complexity enriches the scientific landscape, providing insight into both basic chemical principles and applications with profound societal implications. While hydrocarbons promise energy and material innovation, they also necessitate a conscientious dialogue regarding sustainability and environmental ethics. The fascination with hydrocarbons lies not merely in their structure, but in their implications—dictating chemistry’s role in contemporary challenges while inspiring future inquiry in myriad scientific disciplines.