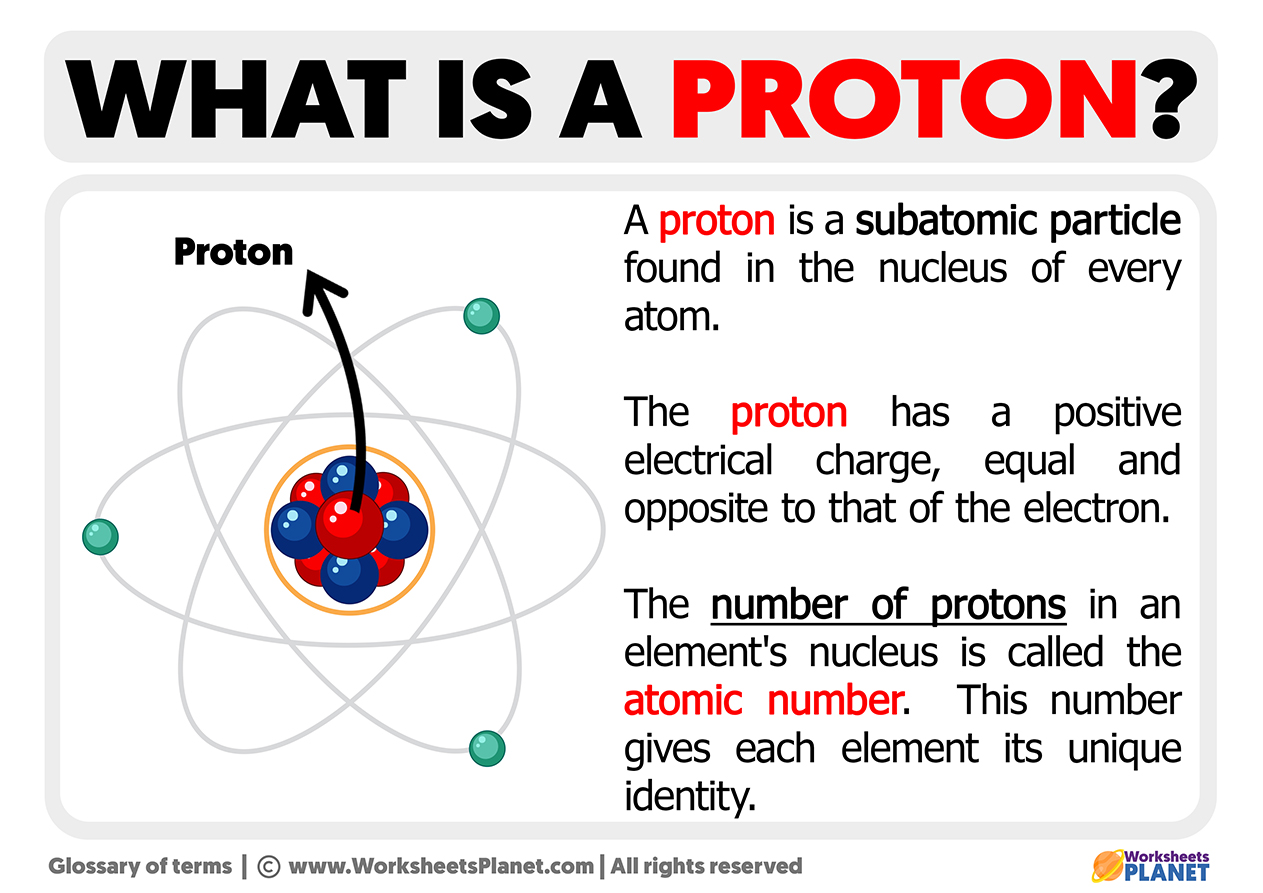
The atom, the fundamental building block of all matter, is primarily composed of protons, neutrons, and electrons. While protons and neutrons are both constituents of atomic nuclei, their relationship is intricate. One might ponder: how can two seemingly distinct entities exhibit striking similarities? In exploring the question of how protons and neutrons are almost the same, we shall delve into their composition, properties, roles within the atomic nucleus, and the implications of their similarities and differences.
At the heart of this inquiry lies the concept of baryons. Both protons and neutrons belong to this category of particles, characterized by their composite nature. They are not elementary particles, but rather, are formed by quarks—elementary particles that are held together by the strong force mediated by gluons. A proton consists of two up quarks and one down quark, while a neutron contains one up quark and two down quarks. This slight variation in quark composition accounts for the differences in charge and, to some extent, mass between the two particles. However, their structural foundation is fundamentally the same, framed by the interactions of quarks and the fundamental forces at play.
Both particles exhibit a similar magnitude of mass, albeit with notable distinctions; a proton is slightly more massive than a neutron. The mass of a proton is approximately 938.3 MeV/c², while a neutron has a mass of about 939.6 MeV/c². This mass difference, while crucial in certain contexts such as β-decay, is minuscule when compared to their overall responsibilities in atomic stability. The inherent similarity in their masses plays a substantial role in the binding energy of nuclei, thus influencing the stability and behavior of atomic structures.
Alongside their mass, protons and neutrons share similar spins. Both possess a spin quantum number of ½, which classifies them as fermions—the particles that follow the Pauli exclusion principle. The particles’ spin contributes to their interactions and arrangements during the formation of nuclei. In fact, in nuclear physics, it’s not uncommon for protons and neutrons to behave interchangeably in the interactions that bind them together. This allows for a dynamic interplay within nuclear forces, giving rise to the stability of atoms across the periodic table.
Furthermore, in the context of nuclear forces, the strong nuclear force operates as a critical binding agent between protons and neutrons. This force is incredibly potent but acts over a very short range. It is responsible for overcoming the electromagnetic repulsion experienced between positively charged protons, thereby fostering the cohesion necessary for forming atomic nuclei. Consequently, protons and neutrons, due to their similar physical characteristics and their partnership in nuclear formation, can collectively be termed nucleons. They exhibit remarkably complementary roles in fortifying the stability of the atomic nucleus.
However, it is imperative to scrutinize their divergence. Protons, with their positive charge, and neutrons, which are electrically neutral, confer varying implications in the realm of atomic interactions. The positive charge of protons directly influences the electromagnetic interactions with electrons, establishing the chemical properties inherent in each element. Conversely, the neutrality of neutrons plays a crucial role in moderating forces within the nucleus, preventing excessive repulsion among protons and ultimately contributing to the force balance necessary for nuclear stability.
This dichotomy unveils the profound significance of protons and neutrons in determining not only the identity of an element but also its isotopic variations. Isotopes, which are atoms of the same element having different neutron counts, manifest in significant ways, influencing atomic stability, reactivity, and the myriad of phenomena observed in nuclear chemistry and physics. The coexistence of varying neutron counts while maintaining a constant proton count highlights the adaptability of atomic structures. Through this lens, one can deduce that, in many ways, protons and neutrons serve a versatile functionality that enhances our comprehension of atomic behavior.
Expanding our vista further, the similarities and differences between protons and neutrons have profound implications in fields such as astrophysics and quantum mechanics. The fusion processes powering stars hinge on the interactions of protons and neutrons, where the delicate balance of forces determines energy release. Additionally, the decay of unstable isotopes, a phenomenon of great interest, exemplifies the transformative capabilities inherent in the slight variations between protons and neutrons. Such processes emit radiation, presenting tangible consequences for various domains including medicine, energy generation, and material science.
Thus, the juxtaposition of protons and neutrons can serve as a lens through which we may investigate the overarching principles governing the subatomic realm. Their collaborative existence underscores the intricate balance required in retaining the universe’s structural integrity. From chemical bonding to nuclear operations, the complementarity and interdependence of these particles are foundational to the principles underpinning not only atomic theory but also the broader tapestry of physical science.
In conclusion, while protons and neutrons exhibit divergent characteristics—most noticeably their charge—they collectively embody the essence of atomic structure. Their shared attributes in mass, spin, and role within the atomic nucleus elevate them from mere constituents to critical agents in the narrative of matter. Embracing their similarities offers not only an enriched understanding of atomic behavior but also sets the stage for exploring the depth of interactions that govern our universe, piquing curiosity and promising a transformative perspective on the nature of existence itself.