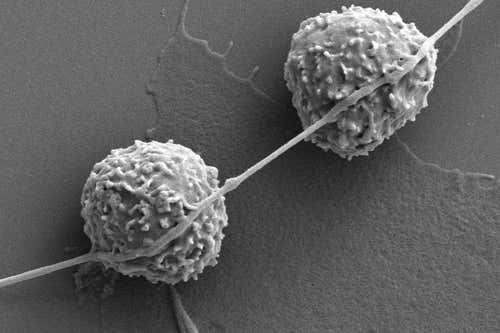
In the tapestry of cellular interactions and behaviors, one might jest: what happens when cellular entities embark on a gastronomic adventure that breaks not just the biological norm, but the very essence of life? A seemingly benign confluence of nanotechnology and cellular metabolism creates an intriguing conundrum: cells consuming nanotubes—with potentially lethal ramifications.
As scientific inquiry advances, it becomes evident that carbon nanotubes (CNTs), recognized for their extraordinary mechanical properties and conductivity, find their way into biological systems through myriad avenues. The juxtaposition of these synthetic structures with biological entities reveals an unsettling narrative. What do cells perceive when they encounter these alien nanostructures? The cellular behavior, particularly in the context of energy acquisition and waste management, is poised on the precipice of evolutionary adaptation and hazardous entanglement.
Carbon nanotubes, with their cylindrical nanostructure, derive their pertinence from the molecular resilience they exhibit. Comprised chiefly of carbon atoms arranged in a hexagonal configuration, they boast remarkable tensile strength and an unrivaled surface area. However, their compatibility—or lack thereof—with biological systems becomes an immediate concern. As stellar as they are for applications in electronics and materials science, what implications arise when these entities transgress into the microscopic realm of the cell?
The phenomenon of cellular ingestion, or phagocytosis, is an intricately choreographed process wherein cells engulf and internalize larger particles. Typically, this process serves defensive mechanisms or nutrient acquisition. However, the ingestion of CNTs introduces an aberration. Cells treat these foreign bodies as potential sustenance, a treacherous misconception. Can one ponder the extraordinary irony: that which was engineered for technological progress could epitomize biological disarray?
Numerous studies shed light on how cells interact with carbon nanotubes and the physiological repercussions of this symbiosis—or, perhaps more aptly, this parasitism. Upon internalization, CNTs evoke inflammatory responses, leading to oxidative stress and cell damage. The delicate balance of cellular metabolism falters, leading to aberrant signaling pathways that govern cell proliferation and apoptosis. Tumor cells, in their relentless pursuit of energy, may exhibit a penchant for these entrapments, utilizing them as malignantly divergent energy sources. The question, then, arises: could this behavior extend the proliferative capacity of malignant cells further into realms of hyperplasia?
Alongside the immune system, crucial to the body’s defense mechanisms, lies an inevitable vulnerability. Cells of the immune system, when confronted with CNTs, wrestle to respond. Their innate propensity to combat invaders becomes bewildered—a misdirection resulting in self-harm. The use of materials that elude immune detection can provide malignant cells with a tactical advantage, ultimately destabilizing homeostasis within the organism. What happens when the very components intended for defense become an unwitting ally to the aggressor?
The beauty of cellular machinery is steeped in adaptability. Yet, this scenario presents a seductive irony. Cells, hardwired for survival and adaptation, may inadvertently foster their own demise. Tumors may exploit this phenomenon, harnessing the energy of captured nanotubes to sustain their unchecked proliferation. A cycle emerges, intertwined with the fabric of malignancy—an unrelenting spiral that evades the checks and balances of normal cellular physiology.
This triggers a vital inquiry: what are the long-term implications of introducing such substances into biological contexts? Nanotechnology promises advances—drug delivery systems, biosensors, and regenerative medicine—but at what cost? Is it conceivable that the benefits could one day manifest as vaulted threats, as our engineered marvels become remnants of a hubristic endeavor?
In navigating the intricate pathways of cellular biology, it is pivotal to examine the roles of toxicity and biocompatibility. As researchers delve into the mechanisms underpinning CNT-induced cellular distress, the exploration could illuminate pathways for remedy or offer a harrowing glimpse into potential devastation. One must ponder: what innovative solutions could we devise to counteract the detrimental effects of these nanotube interactions? Can engineered solutions emerge to mitigate the vast chasm between technology and biology?
As investigations persist and novel findings emerge, the microbial world frequently unveils strategies that could inform our understanding of cellular responses to nanostructures. The advent of synergistic approaches, incorporating materials science with biological engineering, may yield antidotes to mitigate the adverse interactions of CNTs. The juxtaposition of nanotechnology and cellular integrity offers a rich tapestry of exploration—a landscape where peril and promise coexist.
Even further, examination into cellular behaviors illuminates opportunities for targeted therapies using or against such nanotubes. Could we manipulate the properties of CNTs to serve as carriers for therapeutic agents, invoking an engineered alliance rather than a grave miscommunication? What challenges must be surmounted to navigate ethical parameters while harnessing the fruits from this complex entanglement?
In conclusion, the adventurous journey of cellular ingestion of nanotubes, fraught with perils, prompts an intellectual curiosity: how do we reconcile the vacuum between the creation and control of technology in biological realms? While the potential for misuse exists, the promise for profound breakthroughs likewise lingers, urging a responsible stewardship in the dance of cells and scaffolded materials. As the frontier advances, the dialogue between biology and technology shall likely continue to weave itself into the fabric of modern scientific ethos.