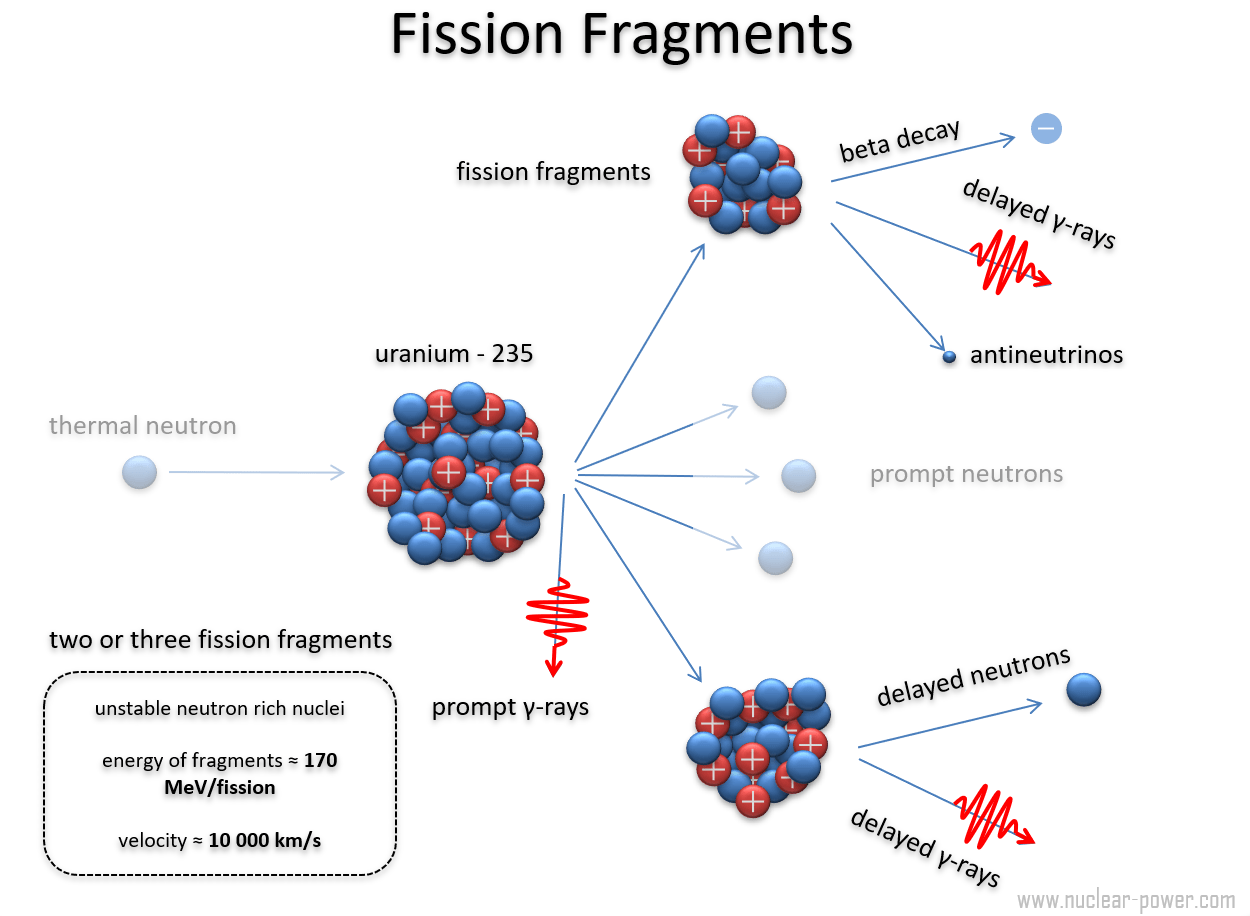
Nuclear fission is a profound phenomenon at the heart of nuclear physics, wherein the nucleus of an atom is split into two or more smaller nuclei, alongside the release of energy. This process not only illuminates our understanding of atomic structure but also underpins the functioning of nuclear reactors and the devastation of nuclear weapons. When an atomic nucleus undergoes fission, it produces a myriad of particle fragments—known as fission fragments—that vary significantly in their properties and behavior. Understanding these fragments is crucial for both scientific inquiry and practical applications.
Fission fragments primarily consist of two types: the primary fission products and secondary products. The primary fission products are the immediate results of the nucleus’s breakdown, while secondary products emerge from the decay of the primary ones. Typically, the energy from fission reactions is enormously potent; in fact, the energy release can be on the order of millions of electron volts (MeV).
When a heavy nucleus, such as Uranium-235 or Plutonium-239, absorbs a neutron and becomes unstable, it may split into fragments. This splitting can yield two or three smaller nuclei, often in the range of 90 to 150 nucleons. The majority of fission products exhibit a mass number concentrated around the mid-range of the periodic table, approximately between mass numbers 80 and 160.
Among the most common fission fragments are isotopes of Krypton, Barium, and Strontium. Krypton-92 and Barium-141 are prime examples. These isotopes are directly emitted post-fission, illustrating the diverse nature of particles produced through this phenomenon. Krypton-92 is an inert gas and does not readily react with other elements, contributing to its persistence in various environments. In contrast, Barium-141, an alkaline earth metal, has a much higher reactivity and participates frequently in subsequent chemical processes.
Furthermore, Strontium isotopes, particularly Strontium-90, hold additional significance due to their biological implications. Strontium-90, a beta-emitter with a half-life of approximately 29 years, mimics calcium and readily accumulates in bones, posing serious health risks through internal radiation exposure during nuclear accidents.
In addition to these primary fission products, neutron emissions accompany the fission event. The emitted neutrons can induce further fission in surrounding fissile material, contributing to a self-sustaining chain reaction, a principle that is ingeniously harnessed in nuclear reactors for energy production.
Another notable aspect of fission fragments involves their radioactivity. Many of the products, such as Iodine-131 and Cesium-137, undergo a process of beta decay, leading to the emission of beta particles and the generation of new isotopes. Iodine-131 has a half-life of about 8 days and is particularly concerning in terms of environmental contamination due to its tendency to concentrate in the thyroid gland, thereby raising the risk for thyroid cancer. On the other hand, Cesium-137 has a longer half-life of around 30 years, leading to prolonged environmental monitoring and remediation efforts in areas affected by nuclear fallout.
The variety of fission fragments can be attributed to the vast combinations of different isotopes formed during fission. These isotopes are categorized as “light” and “heavy” fission products. Light fission products typically have atomic masses of less than 100, while heavy fission products often reside between an atomic mass of 100 and 160. This categorization is crucial for understanding the yield and distribution of the formed fission products, informing both safety protocols in nuclear energy production and strategies for managing radioactive waste.
Moreover, the decay chains following fission are intricately complicated. As fission products decay, they might further transform into heavier elements through a series of alpha and beta decays, resulting in a complex web of isotopes. This decay process not only generates additional radiation but also results in a variety of elemental configurations in the spent fuel, necessitating robust containment strategies and prolonged storage solutions until the radioactivity decreases to safer levels.
Additionally, fission fragments contribute to the observable phenomena in the reactor environment, such as neutron activation and radiation shielding phenomena. The characterizations of neutron flux—comprising thermal, fast, and epithermal neutrons—are critical for optimizing the reactor design and for the deployment of shielding materials that mitigate radiation risks.
In conclusion, the particles emerging from nuclear fission are as diverse as they are scientifically significant. Comprehending the spectrum of fission fragments, including their atomic characteristics, radiological hazards, and interaction with surrounding materials, provides essential insights into nuclear science. The dual nature of fission fragments—as both energy sources and environmental risks—demands meticulous consideration in both reactor design and nuclear safety protocols. The evolving landscape of fission research continues to reveal new fragments and behaviors, shaping a deeper understanding of matter at its most fundamental level.