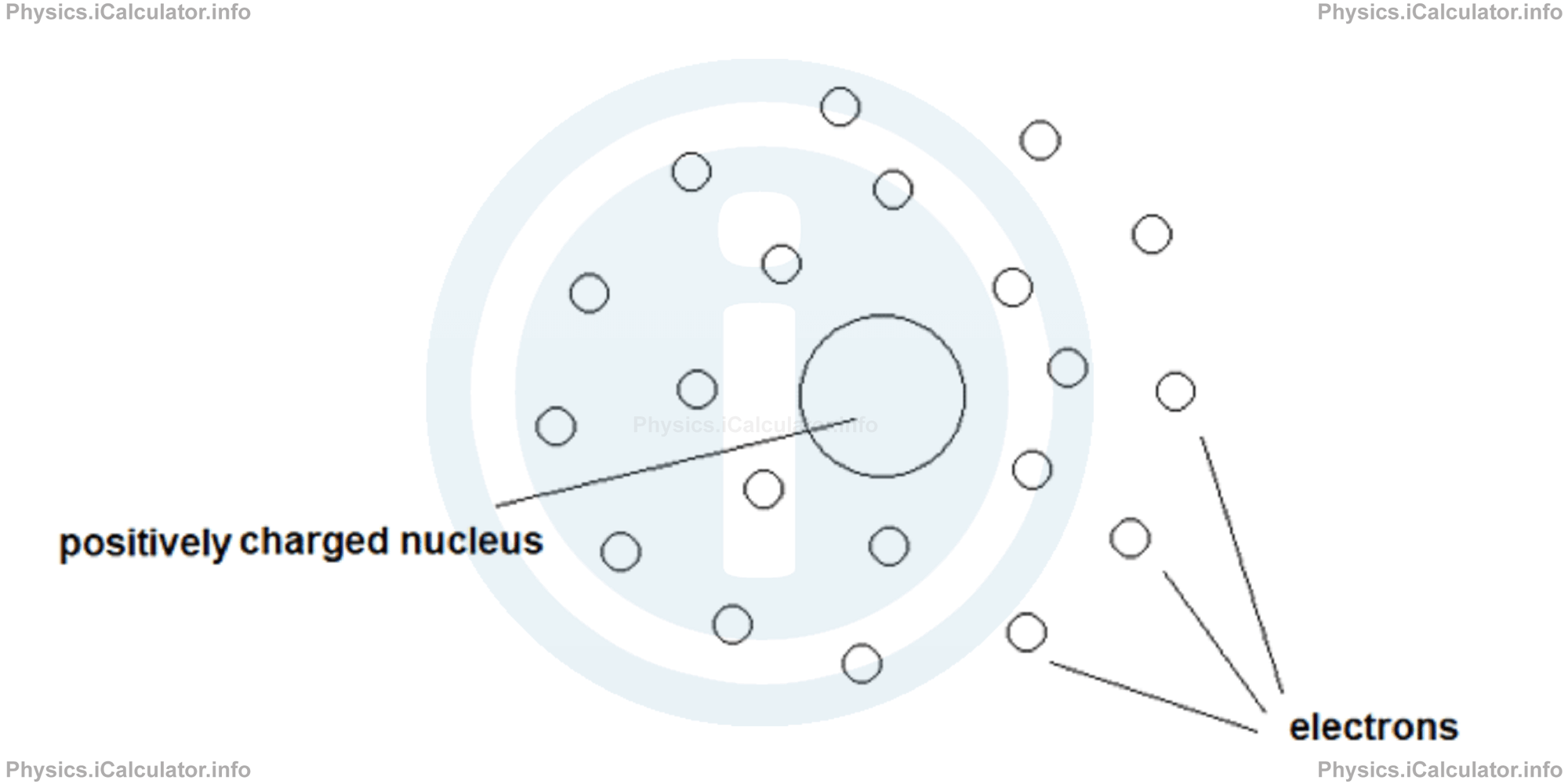
At the dawn of the 20th century, the world of atomic physics was ripe with speculation and inquiry. Many respected scientists held various theories about the structure of the atom. However, a transformative experiment conducted by Ernest Rutherford would irrevocably alter the academic landscape of atomic models. The question arises: how did Rutherford conclude that the nucleus is a positively charged entity? Let us embark on an exploration of his experiment, the implications of his findings, and the broader context of atomic theory.
Rutherford’s groundbreaking experiment, often referred to as the gold foil experiment, was not merely a stroke of serendipity but a meticulously designed investigative endeavor. Conducted in 1909 at the Université de Manchester, Rutherford and his collaborators Hans Geiger and Ernest Marsden aimed to probe the structure of the atom by bombarding a thin sheet of gold foil with alpha particles. These particles, emitted from a radioactive source, possessed significant kinetic energy and were hypothesized to traverse the atom with minimal interaction.
To contextualize the motivations behind this experiment, let us remind ourselves of the prevailing atomic model of the time: Thomson’s plum pudding model. According to this model, atoms were envisioned as a diffuse cloud of positive charge with electrons embedded like raisins. However, as Rutherford sifted through the experimental data, he soon encountered anomalies that prompted a re-evaluation of this model.
Upon examination of the results, Rutherford noted an unexpected phenomenon: while the majority of alpha particles passed through the foil with little deflection, a small fraction were significantly deflected at very large angles. This observation posed a tantalizing puzzle—why would some particles undergo such drastic changes in trajectory? If the atom were predominantly a uniform positive charge, such substantial deflections seemed counterintuitive.
To elucidate this mystery, Rutherford posited that the atom must possess a small, dense core that was positively charged—this core would later be identified as the nucleus. He was compelled to reconcile the data with an innovative hypothesis. When alpha particles approached this densely packed, positively charged nucleus, repulsive forces would come into play, resulting in the dramatic scattering he observed. As he articulated, it was analogous to firing a fast-moving bullet at a large, solid piece of artillery, with the inevitable expectation of ricochet.
The implications of this hypothesis were far-reaching. If the nucleus was indeed responsible for the observed deflections, it could account for the majority of the atom’s mass while occupying an extremely small portion of its overall volume. This stark contrast to the previously accepted models underscored the complexity and dynamism of atomic architecture. Rutherford’s nucleus proposed a microcosm brimming with potential energy and dynamism.
But let us reflect: what challenges did Rutherford face in convincing the scientific community of his conclusions? The allure of the plum pudding model, with its intuitive visual of the atom’s structure, made it difficult for many contemporaries to abandon longstanding beliefs. Science is replete with the tension between established paradigms and innovative ideas. As a physicist, one must consider the innate resistance to change in scientific thought—Rutherford’s challenge was not merely to present data but to cultivate a new understanding of atomic structure that contradicted decades of doctrine.
Further validation of Rutherford’s findings emerged in the subsequent years. Niels Bohr expanded upon Rutherford’s atomic model by introducing quantized electron orbits, incorporating the notion of quantization to describe atomic stability. Bohr’s model seamlessly interwove Rutherford’s nucleus with the behavior of electrons, thus establishing a foundation for modern quantum mechanics. The convergence of these brilliant minds illustrates the collaborative nature of scientific progress, where great ideas often build upon the discoveries of others.
Rutherford’s intellectual legacy extends well beyond the confines of the laboratory; it paved the way for a multitude of explorations into nuclear physics. The realization that the nucleus was not only a physical entity but also a repository of positive charge heralded unprecedented experiments in particle physics, ultimately leading to the discovery of protons and neutrons, transforming our understanding of matter. Yet, it begs the question: how much do we know about the interactions within the nucleus, and what undiscovered phenomena still await our inquiry?
In conclusion, the gold foil experiment decisively established the existence of a positively charged nucleus at the heart of the atom. Through careful experimentation, Rutherford dismantled the prevailing views of atomic structure, ushering in a new era of scientific thought. While it may seem simple now to assert the nucleus’s positive charge, the journey to that conclusion was fraught with intellectual challenge and evolution. Rutherford’s work serves as a testament to the power of empirical investigation and the enduring quest for knowledge in the ever-expanding field of physics.