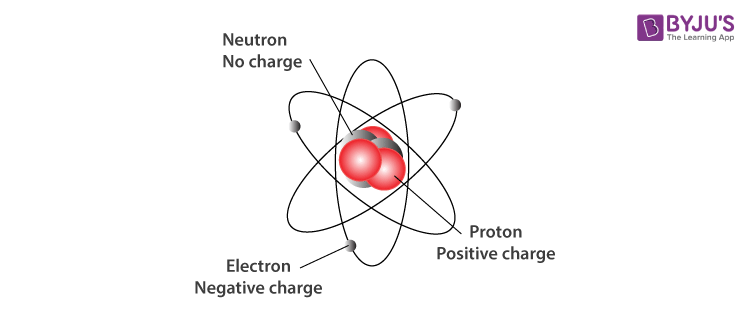
The subatomic world is akin to an intricate tapestry, woven with threads of fundamental particles that together construct the very fabric of matter. Among these threads, three primary subatomic particles—protons, neutrons, and electrons—serve as the keystones of atomic structure and, consequently, the universe itself. Understanding the importance of these particles illuminates our grasp of chemistry, physics, and the overarching phenomena that govern the cosmos.
Protons, with their positive charge, can be likened to the sturdy pillars of a grand edifice. Their presence within the nucleus of an atom dictates not only the identity of the element—each element is defined by its unique number of protons, known as the atomic number—but also fundamentally influences the atom’s behavior and its interactions with other atoms. The positive charge of protons generates an electromagnetic force that attracts negatively charged electrons, thereby establishing the electrostatic foundation upon which chemical reactions occur. Without protons, the existence of atoms as we know them would become untenable, rendering the periodic table a mere figment of theoretical imagination.
Furthermore, protons contribute to the overall stability of atomic nuclei. In a tightly packed nucleus, protons experience repulsive forces due to their like charges. However, neutrons, the unsung heroes of atomic stability, cushion these interactions. Their neutral charge allows them to mitigate the repulsive forces among protons, thus preventing nuclei from disintegrating. This delicate balance between protons and neutrons can be visualized as a well-orchestrated symphony, where each musician plays a crucial role; the harmony achieved results in the robust stability of atoms.
The neutrons themselves carry a wealth of significance. While they may occupy the shadows in the discussions surrounding atomic structure, their contributions are paramount. Neutrons serve as a buffer against the repulsive forces among protons and influence the mass and, thereby, the isotopic identity of atoms. Different isotopes of an element—atoms that differ in the number of neutrons—can exhibit divergent physical properties, leading to a wide variety of applications in scientific research and industry. For instance, the isotope carbon-14, with its distinguishable neutron count, has become synonymous with radiocarbon dating, allowing scientists to unravel the timelines of ancient artifacts and fossils.
The electron, in contrast, represents the more dynamic and elusive component of atomic architecture. Orbiting the nucleus in complex probabilistic patterns dictated by quantum mechanics, electrons are akin to the ethereal dancers in a cosmic ballet, moving with grace and unpredictability. Their behavior is governed by principles such as wave-particle duality and uncertainty, which challenge our classical notions of matter and motion. The intricate electron configurations within atoms dictate their chemical properties and reactivity, forming the basis for the myriad compounds present in nature.
Moreover, electrons play a pivotal role in energy transfer within atomic systems. The absorption and emission of photons during electronic transitions underpin the phenomena of light and color. For example, when an electron in a hydrogen atom absorbs energy, it can transition to a higher energy level; upon returning to its original state, the electron emits light—a principle exploited in numerous technological applications, from neon signs to sophisticated lasers. Thus, the electron not only facilitates molecular interactions but also serves as the conduit for the transmission of energy in diverse systems.
Furthermore, the symmetry and balance achieved through the interplay among protons, neutrons, and electrons reveal a deeper philosophical significance. This triadic relationship mirrors the foundational principles observed across various disciplines, ranging from the dual forces in mechanics to the balance within ecosystems. The importance of subatomic particles transcends mere atomic interactions, reflecting a fundamental order that pervades reality.
In addition to their intrinsic value, the study of subatomic particles leads us to profound advancements in technology and scientific exploration. The development of particle accelerators and colliders, which propel subatomic particles to nearly the speed of light, allows physicists to investigate the fundamental forces that govern the universe. This research paves the way for groundbreaking discoveries—from establishing the existence of the Higgs boson to advancing our understanding of dark matter and energy, the very constituents that shape the cosmos yet elude our sight.
Moreover, the manipulation of subatomic particles has led to the advent of transformational technologies in medical science. The principles underpinning nuclear medicine harness the properties of isotopes, allowing for targeted treatments and diagnostics in oncology. As such, the very understanding of protons, neutrons, and electrons directly translates into life-saving therapies and innovations, demonstrating the practical implications of subatomic particle research.
Ultimately, the significance of protons, neutrons, and electrons within the broader context of matter shapes our understanding of the universe. Their dynamic interplay affords a glimpse into the intricate mechanisms that underpin both the microcosm of atomic structure and the macrocosm of cosmological phenomena. As we further unravel the mysteries surrounding these remarkable entities, we not only deepen our comprehension of existence itself but also embrace the endless possibilities that arise from our knowledge of the fundamental building blocks of nature.