Imagine you were given a puzzle, but instead of simply assembling the pieces into a cohesive image, you find that each piece possesses distinct behaviors and roles within a larger framework. This intricate puzzle is akin to the structure of an atom, the fundamental building block of matter. At the heart of this miniature universe lie subatomic particles, each contributing to the diverse properties of elements. But what exactly are these known subatomic particles, and how do they work together within the atom? Let us embark on this journey to unravel the complexities of these tiny yet crucial constituents of matter.
To begin, it is imperative to understand that subatomic particles can be classified into three primary categories: protons, neutrons, and electrons. However, the intricacies of particle physics extend beyond these familiar entities, leading us to explore additional particles that play pivotal roles in the atomic landscape.
1. Protons
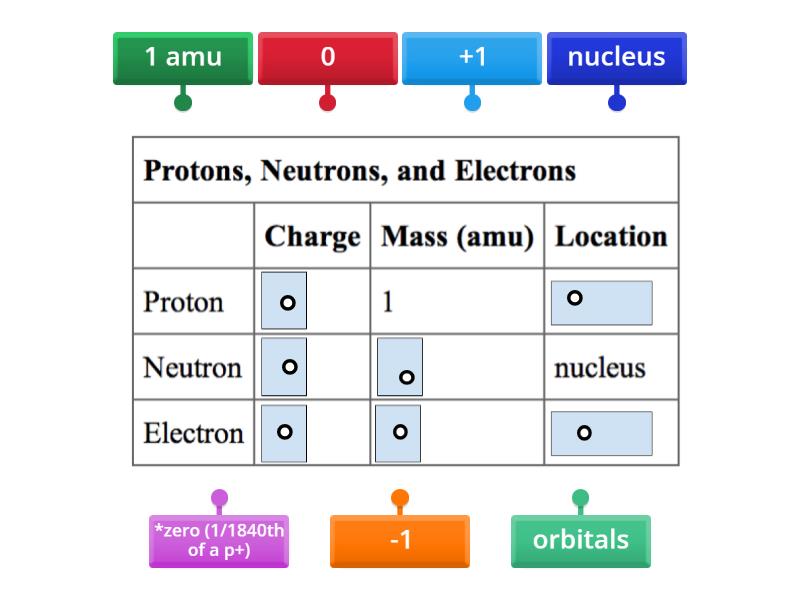
Protons are positively charged particles found within the nucleus of an atom. Each proton possesses a charge of +1 elementary charge and contributes significantly to the mass of the atom. The number of protons is what defines the atomic number of an element, thereby distinguishing one element from another. For instance, hydrogen, with a single proton, is the simplest element, while uranium, with 92 protons, is far more complex and massive. The existence and quantity of protons profoundly influence the chemical properties and reactivity of an element.
2. Neutrons
Neutrons, on the other hand, are neutrally charged particles also located in the nucleus alongside protons. They bear no charge, yet they have a mass nearly equal to that of protons. The presence of neutrons is critical in providing stability to the nucleus; they help mitigate the repulsive forces between the positively charged protons. Interestingly, the number of neutrons can vary for a particular element, giving rise to different isotopes. Isotopes of an element can exhibit varying nuclear stability, impacting their applications in fields such as medicine and archaeology.
3. Electrons
Electrons are the negatively charged subatomic particles that orbit the nucleus at varying energy levels. Each carries a charge of -1 elementary charge, resulting in a natural electrostatic attraction between protons and electrons, which helps to maintain the overall stability of the atom. In a neutral atom, the number of electrons equals the number of protons, balancing the charges. Electrons are responsible for forming chemical bonds—sharing and transferring between atoms, thus shaping the very nature of chemical reactions.
4. Quarks
Diving deeper into the atomic architecture, we discover that protons and neutrons themselves are not indivisible; they are composed of fundamental particles known as quarks. Quarks are elementary particles that aggregate in groups to form protons and neutrons, possessing unique properties such as color charge and fractional electric charge. There are six flavors of quarks—up, down, charm, strange, top, and bottom. The most common combinations, up and down quarks, form protons and neutrons. This composition reflects the profound interconnectedness present in particle physics, emphasizing that even the building blocks of atoms are nested within layers of complexity.
5. Leptons
In addition to quarks, the leptons represent another class of fundamental particles. Electrons are the most well-known leptons, but the family extends to include muons and tau particles, as well as their corresponding neutrinos. Neutrinos are especially elusive, interacting weakly with matter, making them challenging to detect. Despite their diminutive impact on atomic structure, neutrinos play an essential role in nuclear processes, such as beta decay, where a neutron transforms into a proton, emitting a neutrino in the process. The intrigue surrounding neutrinos invites further exploration into their properties and behaviors.
6. Bosons
Now, let us shift our focus to bosons, the mediators of fundamental forces. Bosons are not considered building blocks of matter but are paramount in wielding the forces that govern their interactions. The most recognized boson is the photon, responsible for electromagnetic interactions. Additionally, the W and Z bosons mediate the weak nuclear force, crucial in nuclear decay processes. Furthermore, the Higgs boson, discovered in recent times, is instrumental in endowing particles with mass, adding another layer to our understanding of cosmic composition.
In light of this profound complexity, the exploration of subatomic particles unveils a remarkable interplay of forces and entities. Each particle, whether a proton, neutron, electron, quark, lepton, or boson, plays an indispensable role in the intricate dynamics of atoms. The dance of these particles within the quantum realm challenges our classical intuitions and invites curiosity about the very fabric of reality.
Ultimately, the study of subatomic particles extends far beyond academic curiosity; it profoundly shapes our comprehension of the universe. This microscopic symphony not only elucidates the properties of everyday matter but also underpins the forces that govern the cosmos. As we delve deeper, the question arises: what discoveries await us next in the exploration of the subatomic world? This domain, with its myriad enigmas, is a treasure trove for future physicists and researchers, inspiring awe and igniting passion for discovery. The journey through the subatomic realm is not merely an academic pursuit but rather an invitation to unravel the secrets of existence itself.