Atoms are the fundamental building blocks of matter, revered for their profound role in the composition and behavior of all known substances. Despite their minuscule size, atoms exhibit a tantalizing complexity that captivates the inquisitive mind. Understanding what constitutes an atom is not merely an academic exercise; it is a foray into the intricacies of existence itself. An exploration of the atom unveils a richly structured miniature universe, teeming with interaction and energy, laying the groundwork for the myriad forms of matter we observe in our daily lives.
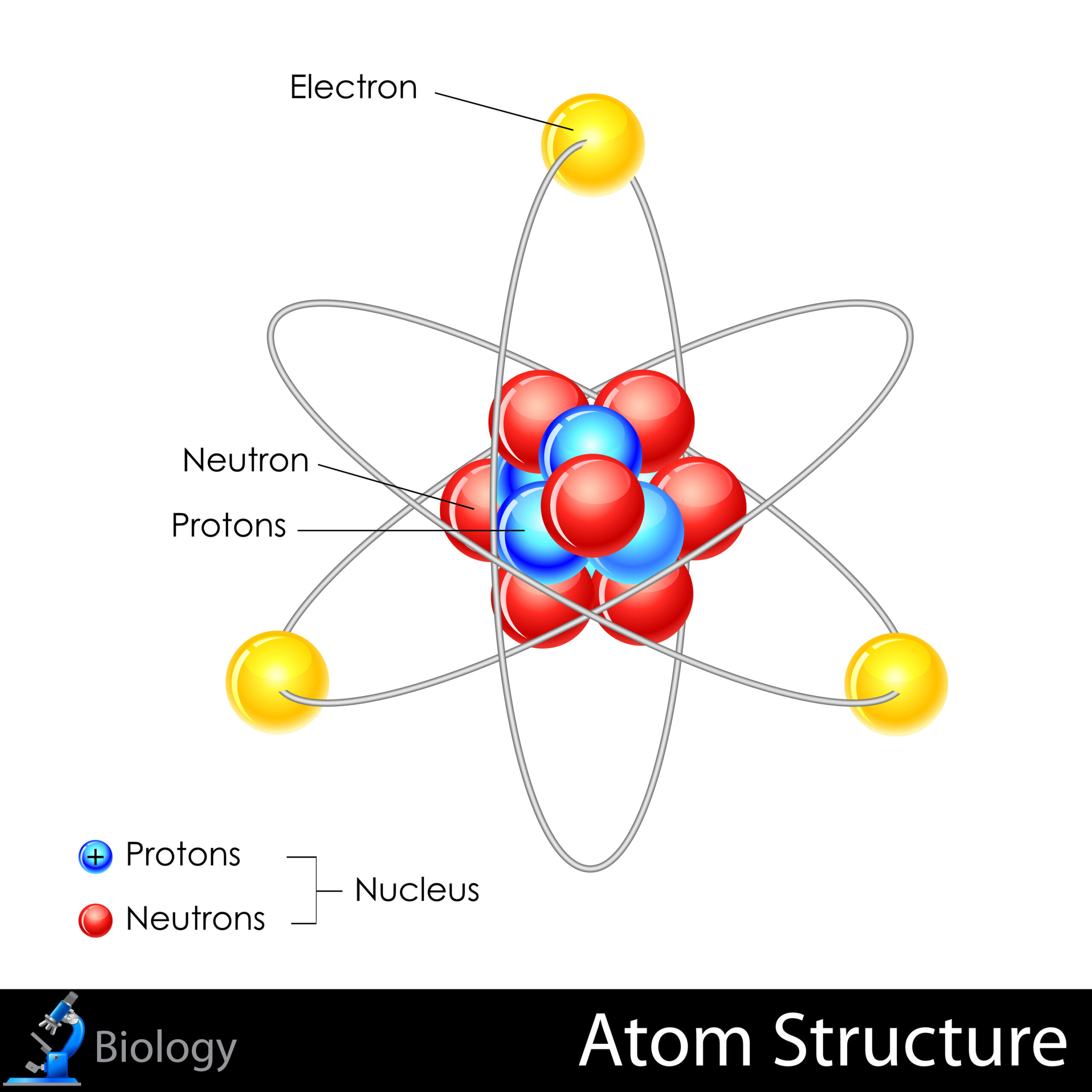
Each atom comprises three primary particles: protons, neutrons, and electrons. Protons and neutrons reside within the nucleus, the atom’s dense core, while electrons inhabit the surrounding electron cloud, engaging in a delicate dance governed by electromagnetic forces. This separation of mass and charge introduces a compelling architecture that is both intricate and paradoxical.
Protons carry a positive charge, with each contributing to the overall mass of the atom significantly. Neutrons, while devoid of electric charge, similarly bolster the atom’s mass. The quantity of protons in an atom’s nucleus, known as the atomic number, determines the element’s identity—for example, hydrogen has one proton, while oxygen contains eight. This atomic identity is not just superficial; it influences elemental properties profoundly, affecting chemical behavior and reactivity.
Electrons, in contrast, are lightweight particles that orbit the nucleus in defined energy levels. Their arrangement is dictated by quantum mechanics, with distinct shells corresponding to variations in energy. Understanding electron configuration is crucial, as it dictates how atoms interact with one another. The outermost electrons, those in the valence shell, are particularly significant as they engage in chemical bonding—the cornerstone of creating molecules and complex substances.
When atoms bond, they may form ionic, covalent, or metallic bonds, each reflecting a unique approach to stability and energy distribution. Ionic bonding occurs when electrons are transferred from one atom to another, typically between metals and nonmetals, resulting in the formation of charged ions that attract each other. Covalent bonding, on the other hand, involves the sharing of electrons among atoms, allowing them to achieve stable electron configurations. Metallic bonding entails a pool of shared electrons, providing metals with their conductive properties.
The interplay between these atomic structures lays the foundation for the macroscopic properties of matter. The collective arrangement of atoms influences material characteristics, such as phase (solid, liquid, gas), density, boiling and melting points, and conductivity. For instance, the rigid lattice of a diamond results from strong covalent bonds between carbon atoms, yielding a material of extraordinary hardness. Conversely, the fluid nature of water arises from weaker hydrogen bonds between molecules, facilitating a liquid at ambient temperatures.
Moreover, the concept of isotopes complicates the atomic narrative further. Atoms of the same element can possess differing numbers of neutrons, giving rise to isotopes that may exhibit unique physical properties or unstable decay patterns, thus enhancing the intriguing diversity of matter. This variability is pivotal in fields such as radiometric dating and nuclear medicine, linking atomic structure with practical applications that affect humanity.
The study of atoms extends into the realms of quantum mechanics, where probabilistic models replace deterministic laws, providing a more nuanced view of electron behavior and atomic interactions. This quantum behavior has paramount implications for understanding the interactions at a subatomic level, leading to phenomena such as quantum entanglement and superposition. These intricate behaviors fuel the burgeoning field of quantum computing, poised to revolutionize technology as we know it.
Furthermore, the importance of atomic interactions is underscored in chemical reactions. The breaking and forming of bonds during these processes drive the transformation of reactants into products. Kiinetic energy, coupled with the principles of thermodynamics, guides these transformations, blurring the lines between various states of matter and enabling complex biological processes necessary for life.
The gravitational interactions among atoms and molecules also play a role in the formation of larger structures, such as stars and planets. The aggregation of atoms under gravitational forces forms celestial bodies, epitomizing the transition from microscopic to macroscopic phenomena. Consequently, an atom’s structure has implications that ripple across the cosmos, showcasing the profound interconnectedness of physical laws.
The richness of atomic composition invokes a deeper fascination, transcending the mere mechanics of particles. It invites contemplation about the essence of existence itself. Our universe, composed of these elementary constituents, reveals a tapestry woven from the intricate relationships between matter and energy. As we probe deeper into atomic structures and interactions, we unveil the beauty of nature’s design, inspiring future explorations in physics, chemistry, and beyond.
To summarize, atoms embody a remarkable construction of protons, neutrons, and electrons, intricately orchestrating the behavior of matter in our world. Their interactions, governed by a diverse range of forces and principles, form the basis for the phenomena we observe, experience, and harness in various scientific and technological ventures. Delving into the composition of atoms and their effects on matter not only informs our understanding of the material world but also inspires a sense of wonder regarding the fundamental nature of reality itself.