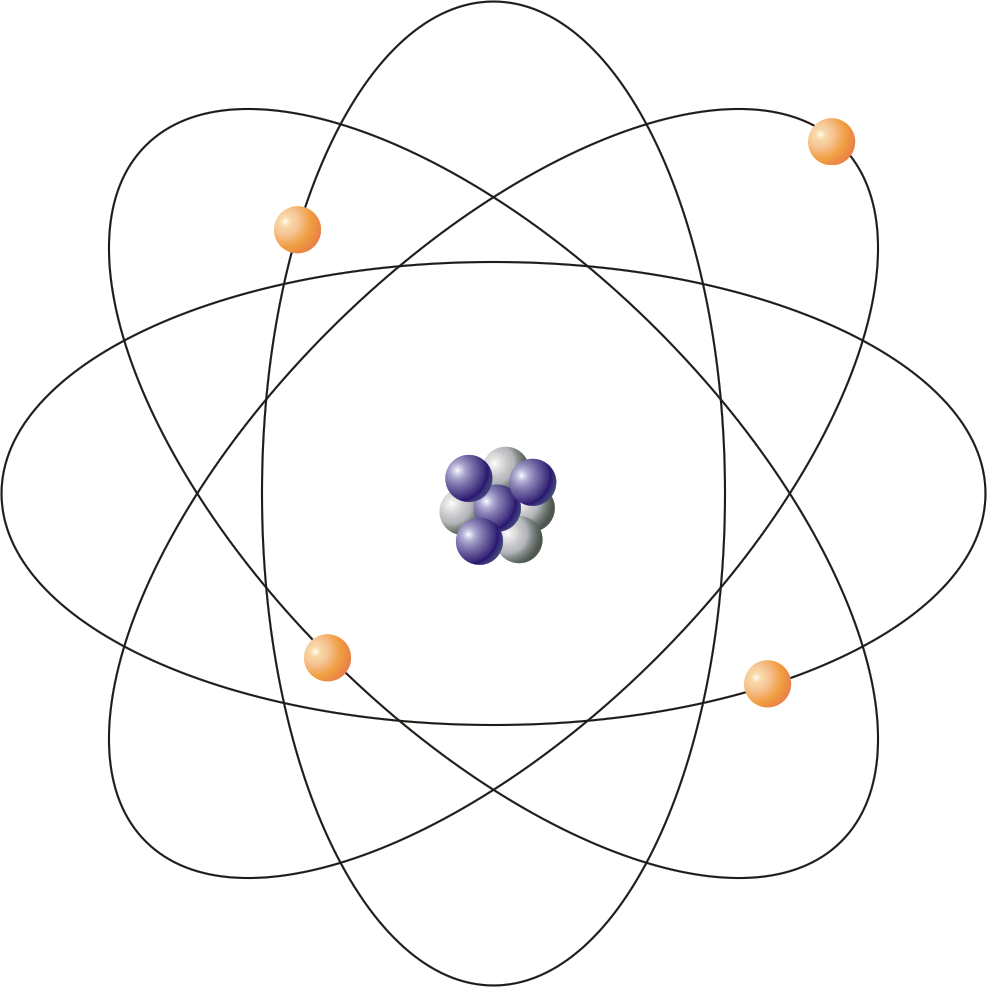
An atom, the fundamental unit of matter, serves as the cornerstone of various scientific disciplines, ranging from chemistry to physics. Analyzing the atom’s intricacies unveils the complex tapestry of the physical universe. To appreciate the atom fully, one must explore its definition, structure, the historical context of its discovery, its role as the foundational unit of elements, and the distinction between atoms and subatomic particles.
At its core, an atom is composed of a nucleus, enveloped by electrons that traverse probabilistic orbits. The nucleus consists of protons and neutrons, both of which are themselves constituted of quarks. The interaction of these subatomic particles underpins the atom’s stability and its interactions with other atoms. Protons carry a positive charge, while neutrons are electrically neutral. Electrons, conversely, possess a negative charge, creating a balanced triad of particles that defines atomic behavior.
Understanding the atomic structure is vital in distinguishing atoms from the smaller particles that comprise them. The term “smallest particle” can be misleading. Although atoms are the smallest units of an element that retain its unique properties, they are composed of even smaller constituents, such as electrons, protons, and neutrons. In this way, one might argue that the atom is not the smallest particle of matter, but rather the smallest particle that exhibits the characteristic properties of an individual element.
The historical underpinning of atomic theory dates back to ancient Greek philosophers, notably Democritus, who posited that matter is composed of indivisible particles called “atomos.” This conceptualization lay dormant until the early 19th century, when John Dalton reinvigorated the atomic theory through empirical observations. Dalton suggested that each element consists of unique atoms, which combine in defined ratios to form compounds. His ideas laid the foundation for modern chemistry, although it was not until the advent of advanced experimental techniques that the atom’s intricacies were fully unveiled.
In the late 19th and early 20th centuries, scientists such as J.J. Thomson and Ernest Rutherford significantly advanced atomic theory. Thomson’s discovery of the electron challenged the notion of indivisibility proposed by earlier philosophers. He introduced the “plum pudding” model, which depicted electrons embedded within a positively charged “soup.” Rutherford’s groundbreaking gold foil experiment refuted this model, revealing the densely packed nucleus at the atom’s center. This pivotal experiment led to the formulation of the nuclear model, thus altering the trajectory of atomic science.
The modern atomic model has evolved further with the integration of quantum mechanics. Niels Bohr refined Rutherford’s model by introducing quantized electron orbits, which explained the discrete spectral lines observed in atomic emissions. This model, albeit simplified, initiated a paradigm shift in the understanding of atomic behavior. Subsequently, the more sophisticated quantum mechanical model provided insights into the probabilistic nature of electron positioning, encapsulated within electron clouds surrounding the nucleus. It emphasizes that while we can predict an electron’s likely location, its precise position remains inherently uncertain.
Atoms are categorized into various elements, characterized by their unique atomic numbers, which correspond to the number of protons present in the nucleus. The periodic table organizes these elements based on their atomic structure. The diversity of elements arises from variations in the number of protons, neutrons, and electrons, leading to distinct chemical properties. For instance, while hydrogen consists of only one proton, heavier elements like uranium have over 90 protons. This atomic diversity allows for an extensive array of compounds, which form the basis of biological and chemical processes.
Furthermore, a profound aspect of atoms is their ability to bond with one another. The chemical bonds formed between atoms—ionic, covalent, and metallic bonds—dictate the properties of the substances they create. For instance, the strong covalent bonds in diamond confer its durability, while the weaker van der Waals forces in graphite give it its lubricating properties. Understanding these interactions is essential, as they are fundamental to fields such as materials science, biochemistry, and pharmacology.
Given the intricacies surrounding atomic structure and bonding, one must also consider isotopes—variants of elements that possess the same number of protons but differing numbers of neutrons. These isotopes can have significant implications in fields such as radiocarbon dating, nuclear medicine, and the study of chemical reactions. The variability in neutron count contributes not only to the stability of a nucleus but also influences the ways in which atoms interact with each other.
In conclusion, the atom stands as a monument of complexity, a fundamental unit embodying the essence of matter. While it serves as the smallest particle of an element, it is not the smallest entity feasible; it encapsulates a myriad of subatomic particles that engage in intricate interactions under the forces dictated by physics. Through historical evolution and scientific inquiry, the understanding of atomic structure elucidates the foundations of chemistry and physics, paving the way for advancements that propel human knowledge further into the realms of science. The exploration of the atom not only illuminates our comprehension of the natural world but invites continuous inquiry into the essential building blocks of the universe.