The fundamental inquiry into the mass of atomic versus molecular entities invites an engaging exploration of the very building blocks of matter. At a glance, one might ponder: which possesses more mass, an atom or a molecule? This seemingly straightforward question delves into the intricacies of chemistry and physics, ultimately challenging the reader to rethink common assumptions about mass and composition.
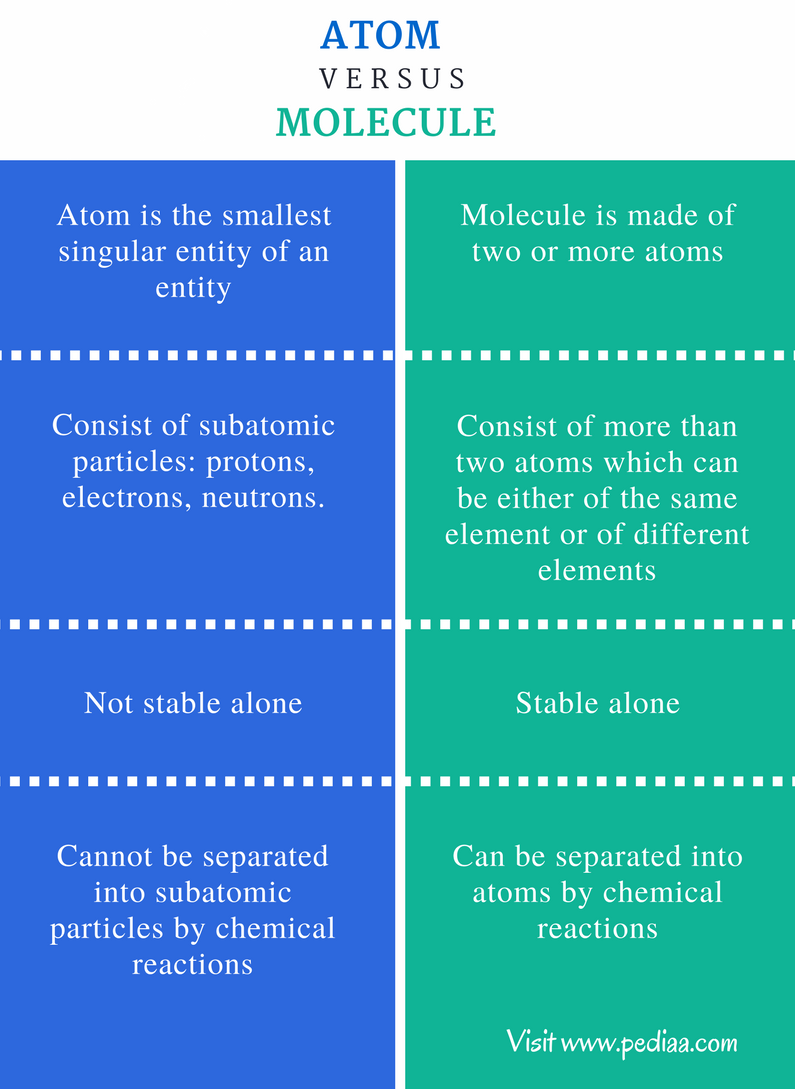
To embark on this intellectual journey, it is crucial first to clarify what constitutes an atom and a molecule. An atom—often referred to as the quintessential unit of matter—comprises a nucleus surrounded by electrons. The nucleus contains protons and neutrons, which account for the majority of the atom’s mass. This inherent structure establishes the atom as a fundamental unit in the realm of chemical elements. In contrast, a molecule is a composite entity formed when two or more atoms chemically bond together. This bonding can occur between identical atoms, as in diatomic molecules like O2, or between different atoms, as seen in compounds such as H2O.
To dissect the nuances of mass, one must examine atomic mass units (amu), which are the standard units used to express the mass of an atom or molecule. The atomic mass of an individual atom is determined primarily by the number of protons and neutrons in its nucleus, as electrons contribute negligibly to overall mass. For instance, a hydrogen atom, the lightest element, has an atomic mass of approximately 1 amu, while a carbon atom weighs in at about 12 amu.
Conversely, when we consider molecules, their mass is calculated by summing the atomic masses of the constituent atoms. Thus, a water molecule composed of two hydrogen atoms and one oxygen atom (H2O) has a total mass of about 18 amu (1 amu for each hydrogen and 16 amu for oxygen). This straightforward arithmetic illustrates a key difference: a molecule, being a collective of atoms, generally possesses a greater mass than any individual atom it comprises.
For further elucidation, it is beneficial to examine various molecular structures. Take, for example, a simple molecule like methane (CH4). Comprised of one carbon atom (12 amu) and four hydrogen atoms (4 amu total), methane’s mass totals 16 amu. In this case, while the individual carbon and hydrogen atoms convey limited mass on their own, their combination yields a significantly heftier molecular identity.
However, the relationship between individual atoms and their molecular counterparts can vary based on the elements involved. Some elements form larger and more complex molecules, leading to even greater cumulative mass. Polymers, such as polyethylene, which consists of repetitive units of ethylene (C2H4), can amass considerably higher masses, illustrating how molecular assembly impacts overall weight.
This compositional complexity brings to light a playful challenge: Can one conceive of scenarios where the differentiation in mass between an atom and a molecule might not be so intuitive? Consider the noble gases—elements such as neon or argon, which exist as singular atoms in nature. When in molecular form, these gases typically occur as diatomic or polyatomic entities only under specific conditions. However, within typical atmospheric contexts, their atomic representation prevails, showcasing how environmental factors may influence mass representations.
Another engaging thought experiment involves isotopes, variants of an element that hold different neutron counts. For instance, carbon exists as both carbon-12 (with 6 neutrons) and carbon-14 (with 8 neutrons). Thus, mass discrepancies in isotopes can challenge the conventional wisdom regarding which has more mass when contemplating the relative atomic weights that feed into molecular formations. Upon inclusion into structures, such variations can materially influence the characteristics of compounds, thereby exemplifying the profound complexity of molecular chemistry.
Furthermore, while the mass of a molecule—especially in biochemical contexts like enzymes or proteins—may denote advantages, it is critical to recognize that molecular attributes extend beyond mere mass. The resulting interactions and reactivity of molecules are defined as much by their structural configurations and electronegativity as by their mass. This depth invites additional considerations regarding thermodynamics and intermolecular forces.
In summation, the overarching verdict remains clear: a molecule typically possesses greater mass than any single atom comprising it. Yet, this conclusion is underscored by may layers of complexity that enrich our understanding of atomic and molecular characteristics. The typical expectation that atoms are the fundamental ‘units’ of mass belies the intricate relationships formed through molecular bonding. Thus, while the answer to the dilemma about mass distinctions may stand resolute, the questions derived from this inquiry can stimulate much deeper dialogues about the nature of matter itself.
Ultimately, the journey through the atomic and molecular landscapes reveals a plethora of complexities that challenge our perceptions. Such inquiries serve as a reminder that science thrives not solely on definitive answers but also on the intrepid exploration of questions that lead to greater understanding and appreciation of our universe.