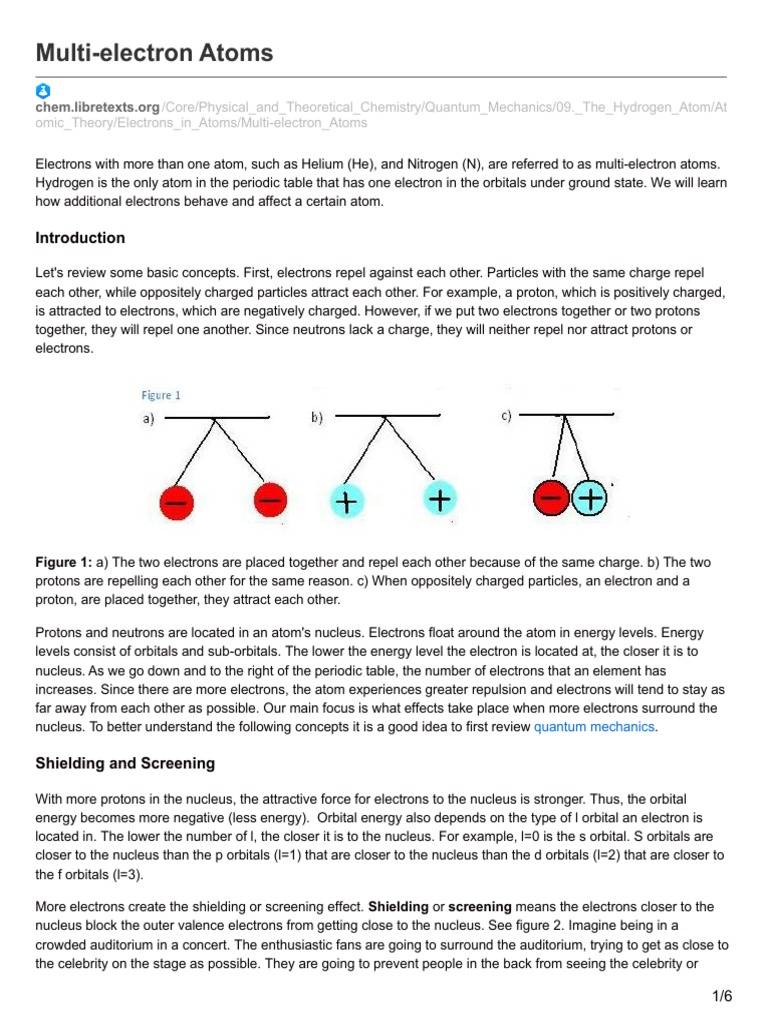
Who explains the spectra of a multi-electron atom? At first glance, this question may seem straightforward; after all, spectra are fundamental to understanding how atoms absorb and emit light. Yet, the answer is laden with complexities, and therein lies an intellectual challenge. Multi-electron atoms present a convoluted interplay of various factors that underlie their spectroscopic properties, providing a fertile ground for exploration within the realms of quantum mechanics and atomic theory. This article unfolds the intricate tapestry of multi-electron atom spectra, elucidating the contributions of various physicists and theories that have paved the way toward our contemporary understanding.
To appreciate who explains the spectra of multi-electron atoms, one must first grasp the underlying principles of atomic structure. In contrast to hydrogen, a single-electron atom whose spectral lines can be precisely calculated using relatively simple quantum mechanical equations, multi-electron atoms introduce a plethora of complications. Each additional electron not only contributes its own set of energy levels but also interacts with the other electrons, leading to phenomena such as electron-electron repulsion, exchange effects, and screening. These interactions create a necessity for a more sophisticated quantum mechanical framework, making the task of predicting spectral lines increasingly challenging.
The pioneering work of Niels Bohr in the early 20th century provided a foundational understanding of atomic structure. His model, which successfully described the hydrogen atom, paved the way for investigating more complex entities. Yet, Bohr’s approach fell short when applied to multi-electron systems; his mathematics could not account for the inter-electronic forces. This necessitated a shift in approach, leading to the advent of quantum mechanics, notably through the contributions of Erwin Schrödinger and Werner Heisenberg. In particular, Schrödinger’s wave equation became a keystone in understanding atomic orbitals and energy levels of multi-electron atoms.
However, even Schrödinger’s equation requires modification to cope with the intricacies of electron interactions. The inclusion of the Pauli Exclusion Principle, articulated by Wolfgang Pauli, further complicates the theoretical landscape. Pauli posited that no two electrons can occupy the same quantum state, leading to the concept of electron configuration. The result is a complex arrangement of electrons characterized by distinct energy levels corresponding to various subshells and orbitals. These configurations are integral to predicting the spectra of multi-electron atoms, as they dictate the possible transitions during electronic excitations and consequently the wavelengths of emitted or absorbed light.
Yet another dimension in this intricate puzzle is the necessity to consider electron correlations. In multi-electron systems, the behavior of one electron is invariably influenced by the presence of its neighbors. Traditional methods, such as the Hartree-Fock approximation, although useful, fail to fully encapsulate these correlations. The Hartree-Fock method approximates the many-body problem by considering mean field effects, leading to a comprehensive but still limited view of multi-electron systems. To address the inadequacies of this approach, more sophisticated theoretical frameworks like Configuration Interaction (CI) and Coupled Cluster (CC) methods have been developed, providing more accurate predictions of atomic spectra.
It is crucial to acknowledge the significant role of spectroscopy in empirically validating theoretical frameworks. The interpretation of spectral lines—both line emissions and absorptions—serves as a test for our understanding of multi-electron systems. When a multi-electron atom is subjected to an external electromagnetic field, the transitions between energy levels can be observed spectroscopically. These empirical observations lend credence to the complex theories developed by physicists over the decades. Indeed, the very arsenal of techniques available today, from laser-induced fluorescence to high-resolution spectroscopy, has propelled the study of atomic spectra into a new era, refining both theoretical postulates and experimental practices.
In the modern era, spectral analysis of multi-electron atoms has proven instrumental in various fields, ranging from astrophysics to condensed matter physics. Astrophysicists routinely employ these spectra to derive elemental compositions of celestial bodies, uncovering the universe’s constituents far beyond our terrestrial horizon. Similarly, condensed matter physicists investigate electron arrangements in materials, linking atomic spectra to macroscopic properties. Here, the explanatory framework expands to encompass not just atomic physics but the intersection of multiple disciplines, underscoring the universality and significance of electron spectra.
One may wonder: can we ever fully understand the spectra of multi-electron atoms? The reality is that while our theoretical approaches continue to evolve, the complexity inherent in these systems suggests that complete understanding may elude us for the foreseeable future. The interplay of quantum effects, inter-electronic correlations, and empirical observations forms an eternal challenge for scientists. Each discovery uncovers new layers of knowledge, yet also reveals further enigmas.
In conclusion, explaining the spectra of multi-electron atoms is a task woven from the contributions of many theoretical and experimental physicists throughout history. While numerous theoretical frameworks exist that can elucidate aspects of their spectra, the continuous refinement of techniques and theories indicates that the quest for a comprehensive understanding is far from complete. Ultimately, it is this multifaceted nature of atomic spectra that not only poses intellectual challenges but also inspires future research and exploration in the ever-evolving field of atomic and molecular physics.