The atom, often described as the fundamental building block of matter, is an intricate entity that has long fascinated scientists, philosophers, and curious minds alike. This fascination stems not solely from its utility in the material world but also from its complex internal structure, which serves as a gateway to understanding the very essence of the universe. Unraveling the composition and behavior of atomic structures offers profound insights into the nature of existence itself.
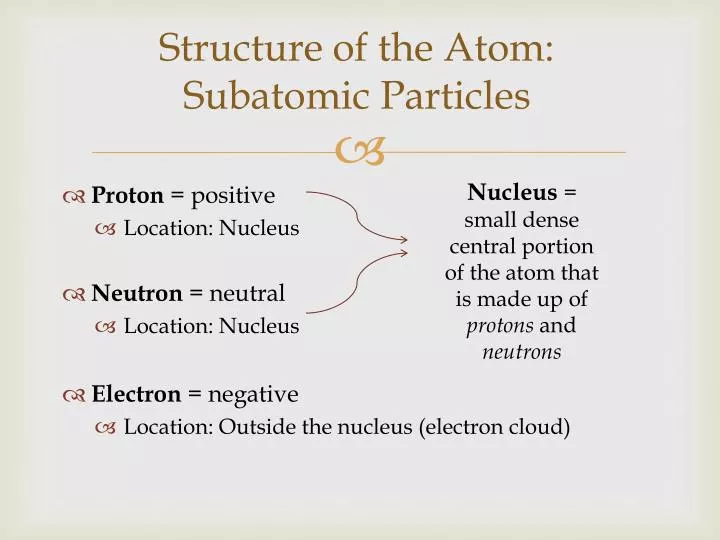
At its core, an atom is comprised of three primary constituents: protons, neutrons, and electrons. These subatomic particles are not merely passive components; rather, they play dynamic and pivotal roles in the chemical and physical properties of matter. Understanding each of these particles is paramount to unlocking the mysteries of the atomic world.
Protons
Protons are positively charged particles located in the nucleus of an atom. Each proton carries a charge of +1 elementary charge, a fundamental unit of electric charge. The number of protons within an atom, known as the atomic number, determines the identity of the element. For instance, a hydrogen atom possesses one proton, while carbon has six. This simple yet profound relationship between the quantity of protons and elemental identity underpins the entire periodic table.
Furthermore, protons significantly influence the atom’s behavior in chemical reactions, as they are integral to the electromagnetic force that governs the interaction between atoms. The strong nuclear force, which is responsible for holding protons and neutrons together within the nucleus, creates an intricate balance with electromagnetic forces evident amongst electrons. This delicate interplay is crucial for the stability of atomic structures.
Neutrons
Neutrons, which are electrically neutral, also reside in the nucleus alongside protons. Their presence brings stability to the atomic nucleus by counteracting the repulsive electromagnetic force experienced by the positively charged protons. Neutrons also play a critical role in isotopic variations of elements. For example, while carbon typically has six protons and six neutrons, an isotope known as carbon-14 contains six protons and eight neutrons, making it vital for applications such as radiocarbon dating.
The number of neutrons in an atom does not affect its chemical identity but profoundly influences its nuclear properties and stability. Atoms with an excess or deficit of neutrons relative to protons may undergo radioactive decay, leading to the emission of radiation and offering insights into both stellar processes and the age of materials. This aspect of neutrons captivates scientists as it opens the door to understanding the forces that govern both atomic stability and the evolution of the universe.
Electrons
Electrons, possessing a minimal mass and a negative charge of -1, orbit the nucleus in various energy levels or shells. The spatial arrangement and distribution of electrons define an atom’s reactivity, bonding behavior, and overall chemistry. Electrons are bound to the nucleus through electromagnetic forces, yet they have enough energy to exist in discrete energy states, which can be quantized. This concept is a cornerstone of quantum mechanics and highlights the wave-particle duality inherent in subatomic particles.
The distribution of electrons around the nucleus is not random; rather, it is governed by quantum mechanical principles. The electron configuration of an atom dictates how it interacts with other atoms. For example, interactions or sharing of electrons during chemical bonding lead to the formation of molecules, which are the substrates of chemical reactions. This ability to bond and form intricate structures reveals a remarkable layer of complexity within atomic interactions, which serve as the foundation of chemistry and material science.
The Uncertainty Principle and Quantum Mechanics
The understanding of atomic structure culminates in the framework of quantum mechanics, which introduces elements of probability and uncertainty in the behavior of subatomic particles. The Heisenberg Uncertainty Principle posits that one cannot simultaneously know an electron’s exact position and momentum. This principle challenges classical notions of determinism and introduces an element of probabilistic outcomes in atomic behavior.
The implications of quantum mechanics extend beyond mere theoretical musings; they manifest in practical applications such as semiconductors, lasers, and even quantum computing. Thus, the atom emerges as not just a static structure but a dynamic entity alive with potential, begging critical inquiry into the forces and interactions shaping our universe.
The Atom’s Role in the Universe
Ultimately, the structure of the atom serves as a microcosm of the universe’s grand tapestry. Each atom, unique in its composition, plays a crucial role in larger systems—from the simplest gases to intricate biological organisms. The understanding of atomic structure contributes to a myriad of fields, including chemistry, physics, biology, and materials science, illuminating the interconnectedness of various disciplines.
As research continues to evolve, the atom remains a central figure in unraveling the mysteries of existence. From quantum realms to cosmic events, the nuances of atomic interactions reveal profound truths about nature. This enduring elegance and complexity instill a sense of wonder about the universe and humanity’s place within it, offering a continuous invitation to explore, understand, and appreciate the delicate interplay of subatomic particles that constitute the very fabric of reality.