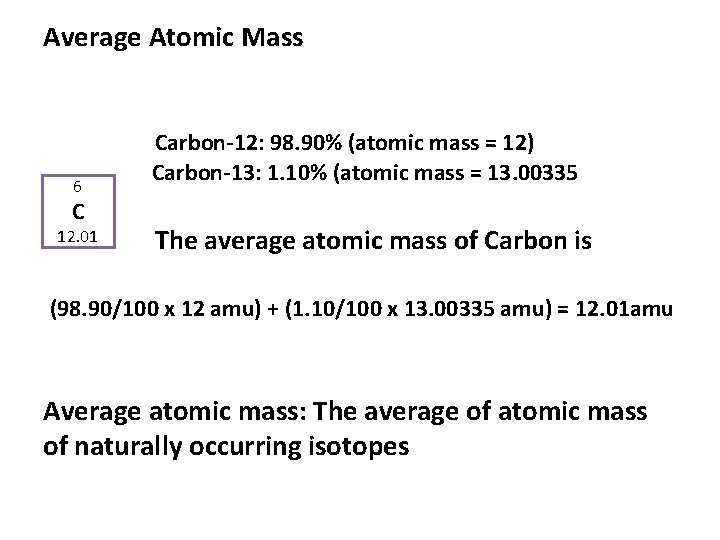
The atomic mass unit (amu), also known as the unified atomic mass unit (u), may seem like a mere speck in the grand cosmos of physical sciences, yet its significance is akin to the proverbial thread that holds the fabric of atomic theory together. Like a delicate yet vital stitch in a grand tapestry, the amu provides coherence to our understanding of matter by establishing a standardized means to express atomic and molecular masses. This article delves into the importance of the atomic mass unit, exploring its crucial role in chemistry, physics, and beyond.
First and foremost, the atomic mass unit serves as the lingua franca of atomic mass measurement. In a world replete with particles, both monumental and minuscule, the amu provides a common platform to discuss and calculate the mass of atoms. Defined as one twelfth the mass of a carbon-12 atom, the amu allows for a simplified comparison of atomic masses across elements. This standardization is crucial in a myriad of applications, from basic chemistry to advanced particle physics. Without the amu, scientists would grapple with chaotic interpretations of atomic mass, leading to inconsistencies and a muddied understanding of atomic interactions.
Further illuminating the significance of the atomic mass unit is its role within the periodic table—a veritable compass navigating the landscape of chemical elements. Each element is emblazoned with an atomic mass, providing insights into not only its identity but also its behavior in reactions. The atomic mass unit facilitates the calculation of molar masses, which are indispensable for stoichiometry, the foundation upon which chemical equations are built. By employing the amu, chemists can accurately predict the outcomes of reactions, balancing equations with the precision of a finely tuned instrument, thereby driving the engine of chemical discovery forward.
The relationship between the amu and isotopes further exemplifies its importance. Isotopes, varying forms of the same element with different neutron counts, can exhibit markedly divergent behaviors in nuclear reactions. The amu not only allows differentiation between these isotopes but also aids in understanding their stability and abundance in nature. For instance, the distinction between hydrogen isotopes—protium, deuterium, and tritium—can be understood through their respective atomic mass units. This understanding proves pivotal in fields such as nuclear chemistry and radiometric dating, where the ages of geological formations or archaeological artifacts may hinge upon minute differences in atomic mass.
Moreover, the atomic mass unit resonates beyond the confines of laboratory benches and academic textbooks; it holds profound ramifications in the realm of astrophysics. The formation of stars, galaxies, and the universe itself is inextricably linked to the processes governed by atomic mass. The creation of elements through nucleosynthesis during stellar evolution relies heavily on the interactions dictated by atomic masses. For example, the synthesis of helium from hydrogen in stars exemplifies how deviations in the amu affect energy production, temperature fluctuations, and ultimately, the lifecycle of celestial bodies. Thus, the significance of the atomic mass unit transcends earthly boundaries, influencing cosmic phenomena.
The integration of the atomic mass unit into chemical calculations is also vital for pharmaceutical development. In drug design, the precise mass of molecules determines bioavailability and efficacy. Pharmaceuticals often possess a narrow therapeutic window, where minuscule changes in dosage—dictated by atomic mass—can result in either therapeutic success or catastrophic failure. The amu, therefore, becomes a cornerstone in the meticulous art of pharmacology, guiding researchers to construct molecules with desired properties that align with the physiological complexity of human biology.
In addition to its practical applications, the atomic mass unit bears philosophical implications regarding our understanding of matter. The very act of measurement elevates the pursuit of knowledge, revealing the intricacies of the universe’s fabric. The dichotomy between the infinitesimally small and grand scales is unified when one contemplates the atomic mass unit. It serves as a bridge—a metaphorical staircase—leading from subatomic particles to the macroscopic world, thus enriching our comprehension of existence itself.
Furthermore, the adoption of the atomic mass unit within international scientific discourse fosters global collaboration. Science thrives on shared vocabulary, and the amu has become entrenched in the vernacular of scientists worldwide. This standardization is pivotal in stimulating research and innovation across multidisciplinary fields, uniting chemists, physicists, biologists, and even environmental scientists in a cohesive effort to unravel the complexities of matter. Such collaboration is essential in our current climate, where transnational challenges such as climate change and public health necessitate comprehensive and collective scientific responses.
In summation, the atomic mass unit is not an abstract notion, confined to the realms of theoretical physics or esoteric chemistry; it is a fundamental building block of our understanding of the material world. Its importance echoes throughout various disciplines, from the microcosmic interactions of particles to the macrocosmic revelations of astrophysics. As the piece that stitches together the grand narrative of atomic theory, the amu invites us to appreciate the intricacies of matter in both its simplicity and complexity. The atomic mass unit, in its subtle power, energizes the quest for knowledge, inspiring future generations of scientists to unearth the secrets of the universe, one amu at a time.