Lead, denoted by the symbol Pb from the Latin “Plumbum,” is a heavy metal that has intrigued scientists and researchers for centuries. This multifaceted element possesses unique characteristics primarily defined by its atomic structure, which is fundamental in understanding its properties and reactivity. To demystify the atomic structure of lead, we must first comprehend its position in the periodic table, its atomic composition, and the implications of its structural characteristics.
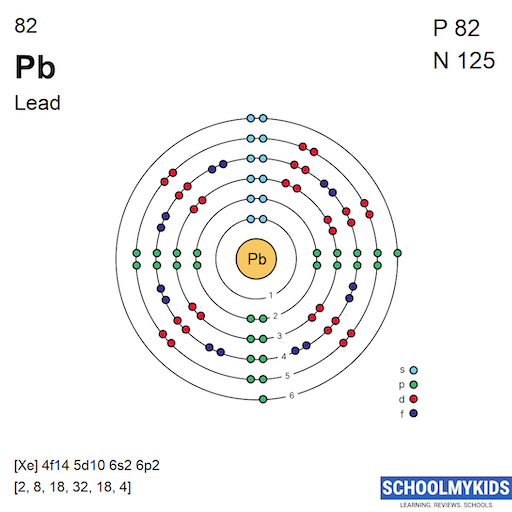
At the core of atomic theory is the concept of atomic number, which for lead is 82. This atomic number signifies that lead has 82 protons nestled within its nucleus. Protons, carrying a positive charge, are pivotal in determining the elemental identity and its place in the periodic table. Accompanying these protons are neutrons, which, although neutral in charge, contribute to the atomic mass and stabilize the nucleus. In lead, the most stable isotope typically contains 126 neutrons, yielding a mass number of 208. This particular configuration encapsulates the essence of lead’s atomic structure.
The electrons, the negatively charged constituents that orbit the nucleus, play an equally critical role in defining the chemical behavior of lead. With 82 electrons, lead’s electron configuration can be expressed as 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s² 4f¹⁴ 5d¹⁰ 6p². This extensive arrangement emphasizes that lead possesses a relatively high number of electrons in its outermost shell, specifically four electrons in the highest energy level (the 6th shell). The presence of these outer electrons sculpts lead’s chemical behavior, particularly its propensity to form bonds.
Delving deeper into the intricacies of lead’s atomic structure, we encounter the concept of electron shells and subshells. Electrons reside in discrete energy levels, or shells, which are divided into subshells (s, p, d, f) that accommodate specific capacities of electrons. The arrangement within these subshells is not just a matter of aesthetics; it fundamentally affects how lead interacts with other elements. The completion of the inner shells (1s, 2s, 2p, etc.) leads to a stable electronic configuration, while the more tenuous outer electrons in the 6th shell play a pivotal role in oxidation states and the formation of compound structures.
Such electron configurations enable lead to exhibit a variety of valence states, which range predominantly between +2 and +4, showcasing its versatility in chemical reactions. This phenomenon of variable valency largely stems from the ability of lead to lose or gain electrons, a trait that aligns with its role as a p-block element situated in group 14 of the periodic table. Understanding lead’s valency allows one to predict its behavior in various chemical scenarios, whether it be in organic compounds, coordination complexes, or metal alloys.
The structural and electronic peculiarities of lead extend into the realm of crystallography. Lead exhibits a face-centered cubic (FCC) crystal structure in its metallic form, which is a manifestation of its atomic packing efficiency and coordination number. The FCC lattice allows for interstitial sites that can accommodate impurities or other elements, thereby allowing lead to form diverse alloys. This structural configuration not only influences the physical properties of lead, such as its density and malleability, but also its thermal and electrical conductivity, properties that can be significantly altered in various alloys.
A compelling aspect of lead’s atomic structure is its relationship with the concept of allotropy. Lead can exist in different structural forms, depending on the temperature and pressure conditions. While most commonly understood in its metallic state, lead can also crystallize into other forms, which may exhibit alternative properties. This flexibility raises questions about the implications of lead’s adaptability in various scientific and industrial contexts—hinting at potential applications or hazards depending on its structural form.
Furthermore, the unique atomic structure of lead can lead to intricate interactions at the molecular level, especially in coordination compounds formed with ligands. Lead’s capacity to form covalent bonds with ligands can result in complexes that are utilized in various chemical reactions. These interactions may also have environmental implications, as lead compounds often play roles in phenomena such as bioaccumulation and toxicity, emphasizing the delicate balance between lead’s advantageous industrial uses and its safety concerns.
The exploration of lead’s atomic structure opens a plethora of inquiries about its functionalities across disciplines. In materials science, knowledge of its fundamental structural characteristics facilitates the development of new alloys with enhanced performance characteristics. In environmental science, understanding how lead interacts on a molecular level helps elucidate pathways for remediation and mitigation of its detrimental effects. Hence, the atomic structure of lead encapsulates far more than mere numbers and configurations; it constitutes a bridge to diverse scientific realms.
In reviewing the atomic structure of lead, it becomes apparent that this heavy metal serves as a paradigm of complexity and adaptability. The interplay of protons, neutrons, and electrons manifests in both its reactivity and physical properties, elevating lead from a simple element on the periodic table to a significant material in various chemical applications. As further studies delve into its atomic structure and behavior, the scientific community stands poised to uncover additional layers of understanding, piquing curiosity and expanding the horizons of material science and chemistry.