In the intricate fabric of the universe, two fundamental building blocks emerge as the primary constituents of matter: atoms and molecules. The distinction between these entities is pivotal in understanding the microscopic canvas upon which the grand tapestry of life and chemical interactions is painted. Hence, we embark on a journey to unravel their meanings, definitions, and the significant roles they play in the realm of science.
1. Defining the Atom
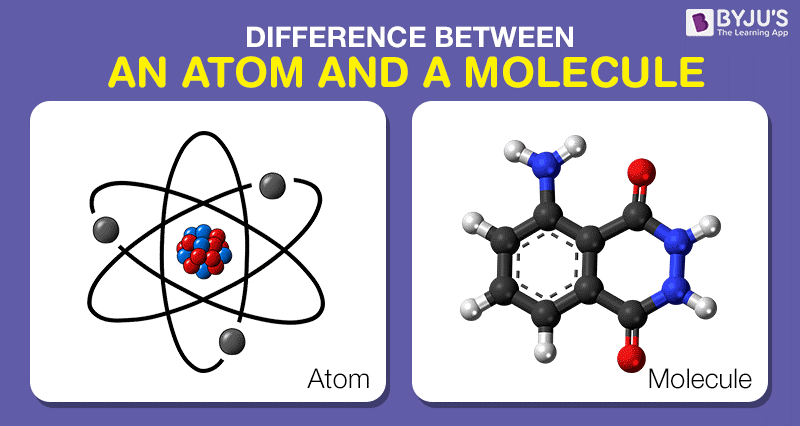
At the most elemental level, an atom is often likened to a singular point of existence, akin to a solitary artist poised with a brush, ready to create. An atom consists of a nucleus, which harbors protons and neutrons, surrounded by a cloud of electrons that orbit in quantized energy levels. The nucleus can be conceived as the heartbeat of the atom, where protons, positively charged entities, and neutrons, neutral particles, create a dense core. The electrons, on the other hand, are the lively dancers that whirl around the nucleus, their arrangements defining the chemical behavior and identity of the element.
Atoms are characterized by their atomic number, which corresponds to the number of protons contained within the nucleus. This simple number serves as the key that unlocks the door to the periodic table of elements, a meticulous catalog that classifies all known elements based upon their atomic structure. Here, each entry tells a story of unique properties, behaviors, and interactions, facilitating profound insights into the nature of matter itself.
2. Understanding the Molecule
In contrast, a molecule can be visualized as an ensemble—a harmonious composition orchestrated from the collaboration of multiple atoms. When atoms bond together, forming covalent or ionic bonds, they conjure molecules, which serve as the agents of chemical reactions. Just as an orchestra creates a symphony from individual instruments, a molecule emerges from the concerted efforts of its constituent atoms, each contributing its unique tonal qualities to the resulting compound.
For instance, a water molecule (H2O) consists of two hydrogen atoms and one oxygen atom, covalently bonded to create a compound imbued with distinctive properties. The nature of bonding, therefore, not only dictates the stability of the molecule but also influences its behavior and interactions in various contexts, from biological systems to atmospheric chemistry.
3. Atoms as the Fundamentals of Elements
The atom stands as the smallest unit of an element that retains its characteristic properties. It is the quintessential identity marker of the substance; thus, carbon is carbon, not merely due to the arrangement of protons but because of its foundational atom. Each element in the periodic table maintains its discretely defined milieu, governed by the interplay of protons, electrons, and neutrons. As one traverses the periodic table, a pattern begins to emerge—a systematic organization that enlightens the relationships between these elemental beings based upon electron configurations and chemical reactivity.
4. Molecules: Complexity and Diversity
Molecules, however, exhibit a complexity that often eludes the simplicity of atomic classification. Compounds formed through the union of different atoms can yield an astonishing array of substances, each with unique physical and chemical properties. Consider the dichotomy of carbon dioxide (CO2) and glucose (C6H12O6); both molecules contain carbon, oxygen, and hydrogen, yet their arrangements and bonds confer radically different functionalities—from contributing to climate change to serving as vital nourishment for life.
The molecular world is often perceived through a lens that captures both structure and function. The shape of a molecule can influence its reactivity and ability to interact with other molecules. This molecular geometry becomes paramount in biological systems, where the three-dimensional structure of proteins and enzymes dictates their ability to catalyze biochemical reactions, positioning the molecule as an active participant in the drama of existence.
5. The Relationship Between Atoms and Molecules
The relationship between atoms and molecules is akin to that of characters within a narrative—a complex interplay that is vital for the unfolding of any storyline. Atoms provide the foundational elements, while molecules tell the story of how these elements combine and interact, giving rise to the variety of substances that compose our world. This synergy between atoms and molecules fosters a rich tapestry of chemical reactions, where energy is exchanged, transformed, and embodied.
6. Conclusion: A Sector of Multidimensional Essence
In the final analysis, atoms and molecules represent the dichotomy between the singular and the collective, the simple and the complex. Atoms serve as the rudimentary identifiers of elements, while molecules transcend individual existence, crafting the richness of materials and life as we perceive it. This relationship underscores the elegance of nature, modeling an integrated cosmos where the interplay of simplicity and complexity generates a pervasive, interconnected reality. The understanding of these constituents not only opens gates to advanced scientific inquiry but invites an appreciation of the orchestrated concert in which every atom and molecule play their distinct and indispensable roles.