Atomic spectroscopy, an intricate tapestry woven from the threads of quantum mechanics and classical physics, serves as a portal into the enigmatic world of atomic structure and elemental composition. Like a prism separating light, this analytical technique dissects the electromagnetic spectrum to reveal the unique fingerprints of various atoms. At its core, atomic spectroscopy hinges on the interaction between electromagnetic radiation and matter, offering profound insights into the constituents of the universe.
The fundamental principle governing atomic spectroscopy lies in the quantized nature of atomic energy levels. Atoms, the building blocks of matter, are composed of a nucleus surrounded by electrons occupying discrete energy states. When energy is imparted to these electrons, through heat or electromagnetic radiation, they can transition between these levels. This phenomenon mirrors the vivid display of a fireworks show—exciting bursts of light illuminating the darkness, akin to electrons jumping to higher energy states before cascading back down, radiating their unique colors. This process of electron excitation and subsequent emission forms the cornerstone of atomic spectroscopy.
Various methodologies exist within the realm of atomic spectroscopy, each with its distinctive nuances and applications. Among the most prevalent are flame atomic absorption spectroscopy (FAAS), inductively coupled plasma atomic emission spectroscopy (ICP-AES), and atomic fluorescence spectroscopy (AFS). Each technique can be likened to a painter’s brush, each stroke producing a different effect while revealing the underlying canvas of atomic behavior.
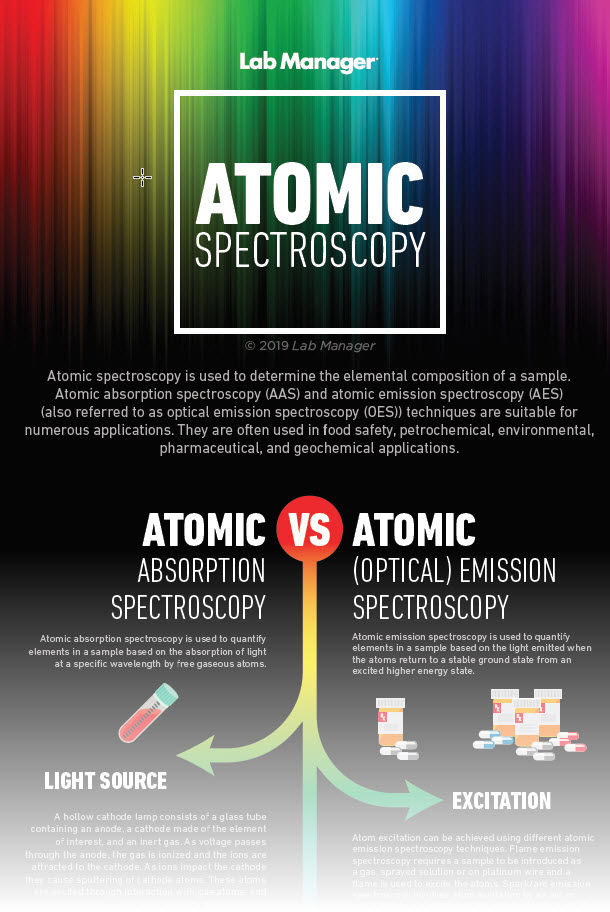
Flame atomic absorption spectroscopy (FAAS) operates on the principle that free atoms absorb specific wavelengths of light, corresponding to their unique electronic transitions. In this process, a sample is nebulized and introduced into a flame, where the heat atomizes the sample, converting it into gaseous atoms. The light from a hollow cathode lamp—emitting a wavelength specific to the element of interest—passes through the flame. The degree to which light is absorbed is proportional to the concentration of the element present, allowing for quantitative analysis. FAAS is akin to a skilled musician tuning an instrument, delicately adjusting to the perfect pitch that reveals the true tone of the elemental symphony.
Inductively coupled plasma atomic emission spectroscopy (ICP-AES) significantly expands the potential of atomic spectroscopy. Utilizing a high-temperature plasma, generated by an inductively coupled radio frequency field, this technique offers a more comprehensive elemental analysis across the periodic table. The sample, once atomized, is introduced into the plasma, where it is subjected to extreme temperatures that excite atoms and ions, leading to their emission of light. The emitted light is then analyzed using spectrometric techniques. ICP-AES acts as a finely tuned telescope, providing astronomers with a glimpse into distant galaxies, revealing the composition of starlight with unparalleled precision.
Atomic fluorescence spectroscopy (AFS), while closely related to its absorption counterpart, diverges in its approach. In AFS, atoms are excited by absorbing incident light, after which they emit light at characteristic wavelengths as they return to a lower energy state. This technique often boasts greater sensitivity than FAAS and ICP-AES, making it particularly valuable in the analysis of trace elements. AFS can be envisaged as an artist’s brush dipped in phosphorescent paint, illuminating the canvas with vibrant colors that only reveal themselves when the light is introduced.
The realm of atomic spectroscopy is not limited to elemental analysis; it also encompasses the nuanced study of isotopes. Isotopic variations of elements provide crucial insights into various processes, from geological formations to metabolic pathways in biological systems. Isotope ratio mass spectrometry (IRMS), for example, leverages the properties of atomic spectra to elucidate the origins of substances by examining the ratios of isotopes present. This technique exemplifies the art of deduction, akin to a detective piecing together clues to reconstruct a narrative from minute evidence.
One cannot overlook the industrial applications of atomic spectroscopy, which span numerous fields including environmental monitoring, pharmaceutical development, and materials science. In environmental studies, atomic spectroscopy serves as a sentinel, enabling the detection of trace contaminants in air, water, and soil. In pharmaceutical contexts, it aids in the characterization of drug compounds and ensures compliance with safety standards, acting as the vigilant gatekeeper of public health. In materials science, the precise composition analysis facilitated by atomic spectroscopy informs the development of new materials with tailored properties, enhancing technological innovation.
The philosophical implications of atomic spectroscopy extend beyond mere analysis; they venture into the realm of existential inquiry. By elucidating the elemental makeup of substances, it challenges our understanding of what constitutes matter and invites contemplation of the very nature of reality. In this sense, atomic spectroscopy becomes more than an analytical tool—it transforms into a profound dialogue between science and philosophy, revealing the intricate designs of atomic existence.
In conclusion, atomic spectroscopy stands as a testament to humanity’s relentless pursuit of knowledge. Like a masterful symphony, it intertwines various elements—energy transitions, light absorption and emission, and profound industrial applications—into a cohesive narrative that unveils the universe’s secrets. Through this lens, one can appreciate not only the utility of atomic spectroscopy in practical terms but also its role as an intellectual endeavor that challenges our understanding and appreciation of the fundamental components of the cosmos.