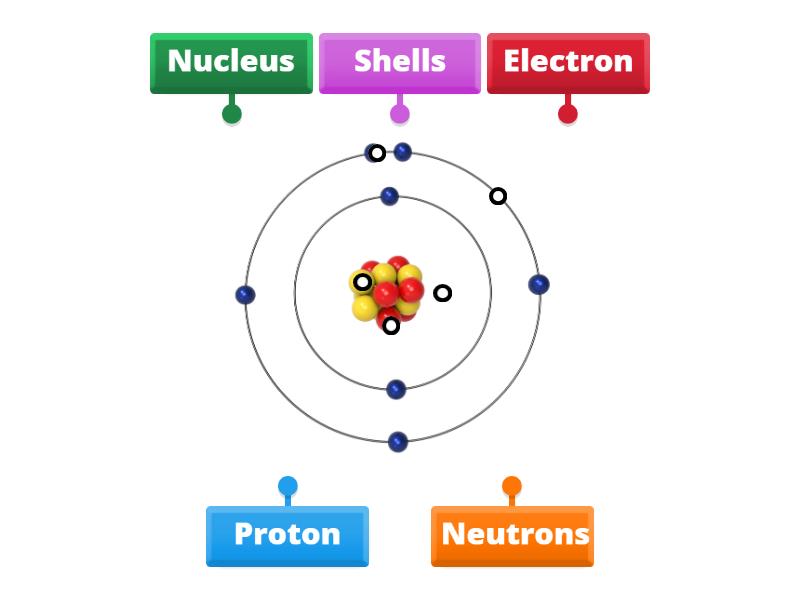
Delving into the enigmatic world of atomic structure, one might ponder: What governs the very essence of matter? What minute constituents make up the nucleus, that tenacious core at the heart of every atom? This inquiry leads us to explore the subatomic particles nestled within the nucleus—protons and neutrons—each playing pivotal roles in defining not only the composition of elements but also the fundamental forces of nature.
To understand the subatomic particles within the nucleus, it is instrumental to first grasp the concept of the atom itself. An atom is the quintessential unit of an element, retaining its distinctive properties. Encased within the nucleus are protons, exhibiting a positive charge, and neutrons, which are electrically neutral. Collectively, these particles account for the majority of an atom’s mass. However, what challenges do these particles present, and how do they interact to shape the larger frameworks of matter and energy?
Let us commence with protons. Indeed, the proton is a cornerstone of atomic identity. Each element is uniquely defined by the number of protons it possesses, a quantity known as the atomic number. For example, hydrogen, with its singular proton, stands as the simplest and most abundant element in the universe. Conversely, uranium, replete with 92 protons, represents one of the heaviest naturally occurring elements. This diversity underscores the significance of protons in the periodic table’s architecture.
But what about neutrons? These stalwart particles add to the atomic nucleus’s heft without bestowing any electric charge. While they may seem elusive in their neutrality, they are essential in stabilizing the nucleus. Without neutrons, the electrostatic repulsion between the positively charged protons would result in nuclear disintegration, unraveling the very structure of matter as we know it. Hence, the neutron’s role is decidedly more nuanced; it acts as a balancer, resisting the forces that would otherwise fracture atomic integrity.
Yet, one cannot discuss the characteristics of protons and neutrons without acknowledging their composite nature. Both particles are classified as baryons, a family of particles that consist of three quarks bound together by the strong force, mediated by gluons. Protons contain two ‘up’ quarks and one ‘down’ quark, whereas neutrons consist of one ‘up’ quark and two ‘down’ quarks. This intriguing substructure invites numerous questions. What governs the behavior and interaction of these quarks? How do the fundamental forces dictate their properties?
The strong nuclear force, one of the four fundamental forces of nature, emerges as a principal player in this atomic drama. This force is responsible for the binding of quarks within protons and neutrons, as well as the binding of protons and neutrons within the nucleus. Its potency is formidable, operating over very short distances, and it overcomes the repulsive electromagnetic force between protons. The residual effects of the strong force provide an additional layer of attraction that keeps the nucleus intact.
However, the interplay of protons and neutrons is not without its complexities. As atomic mass increases, the challenge of nuclear stability escalates. Larger nuclei demand a greater number of neutrons to mitigate the repulsion between protons. This phenomenon leads to isotopes—variants of elements that contain the same number of protons but differing numbers of neutrons. The existence of isotopes is a pragmatic demonstration of nuclear flexibility and stability, yet it also poses challenges in terms of radioactivity and nuclear decay.
Indeed, the story of subatomic particles does not conclude with nucleons. Gluons, the force carriers of the strong nuclear force, also deserve mention. These massless excitations are crucial in the binding mechanism of quarks within baryons. Without gluons, quarks would not cohere to form protons and neutrons, resulting in an entirely different atomic landscape. Thus, the intricate lattice of interactions between quarks, gluons, protons, and neutrons unveils an underlying complexity that transcends mere particle identification.
As we navigate through this quantum realm, one must consider the broader implications of our understanding of subatomic particles. The revelations regarding the structure of the nucleus have profound ramifications for numerous scientific fields, such as quantum physics, chemistry, and even cosmology. For instance, the nuclear processes occurring within stars elucidate not only the genesis of elements but also the very evolution of the cosmos itself.
In contemplating these fundamental particles, one might ask: how does our perception of matter itself shift in light of these insights? Does this advancement in understanding compel us to reevaluate the very nature of reality? The exploration of subatomic particles challenges our intellectual frameworks and invites us to confront the unknown. The quest for knowledge in this domain is intrinsic to the human experience; it embodies our drive to decipher the universe’s mysteries.
Ultimately, the nucleus, brimming with protons and neutrons—the stalwarts of atomic structure—stands as a testament to both the simplicity and complexity of matter. Armed with quantum mechanics, we inch closer to unraveling the tapestry that is the subatomic world. The journey fosters not only scientific inquiry but also a philosophical quest, marking our enduring curiosity about the universe and our place within it.
As a final challenge, consider the implications of these findings. What might the future hold regarding our understanding of nuclei and particles? How could advancements in technology and research unveil further secrets lurking in the subatomic realm? The answers may yet alter our conception of existence itself.