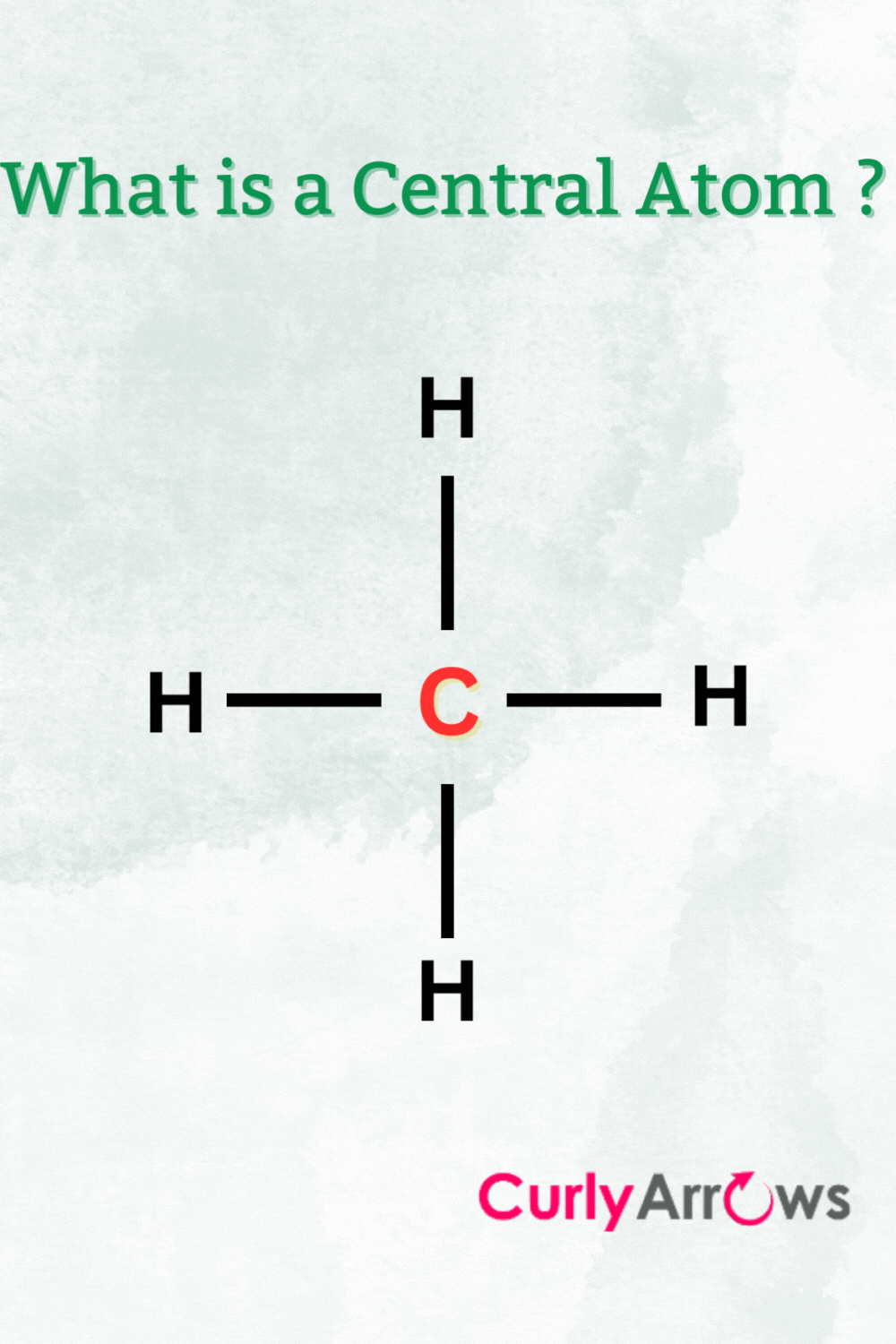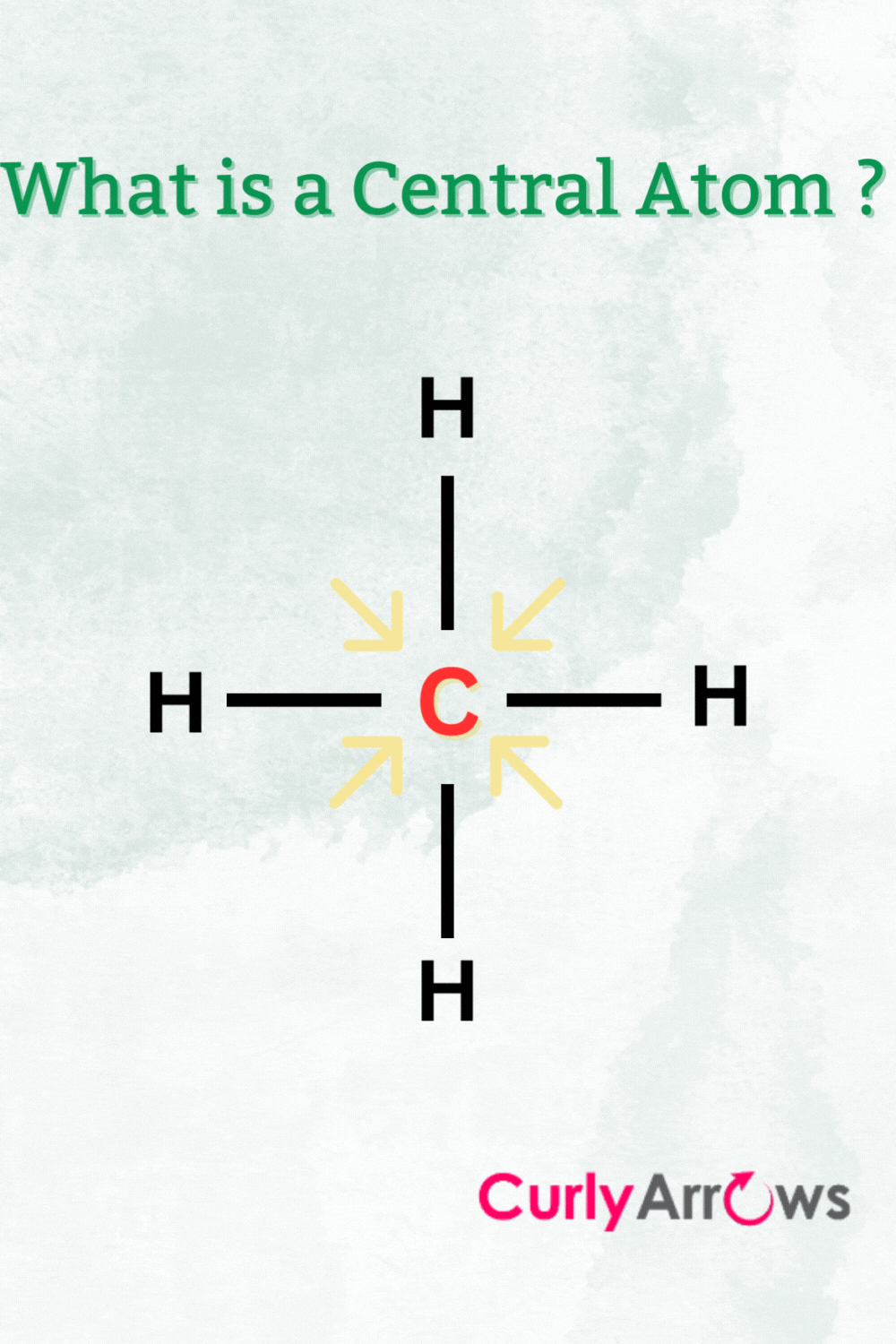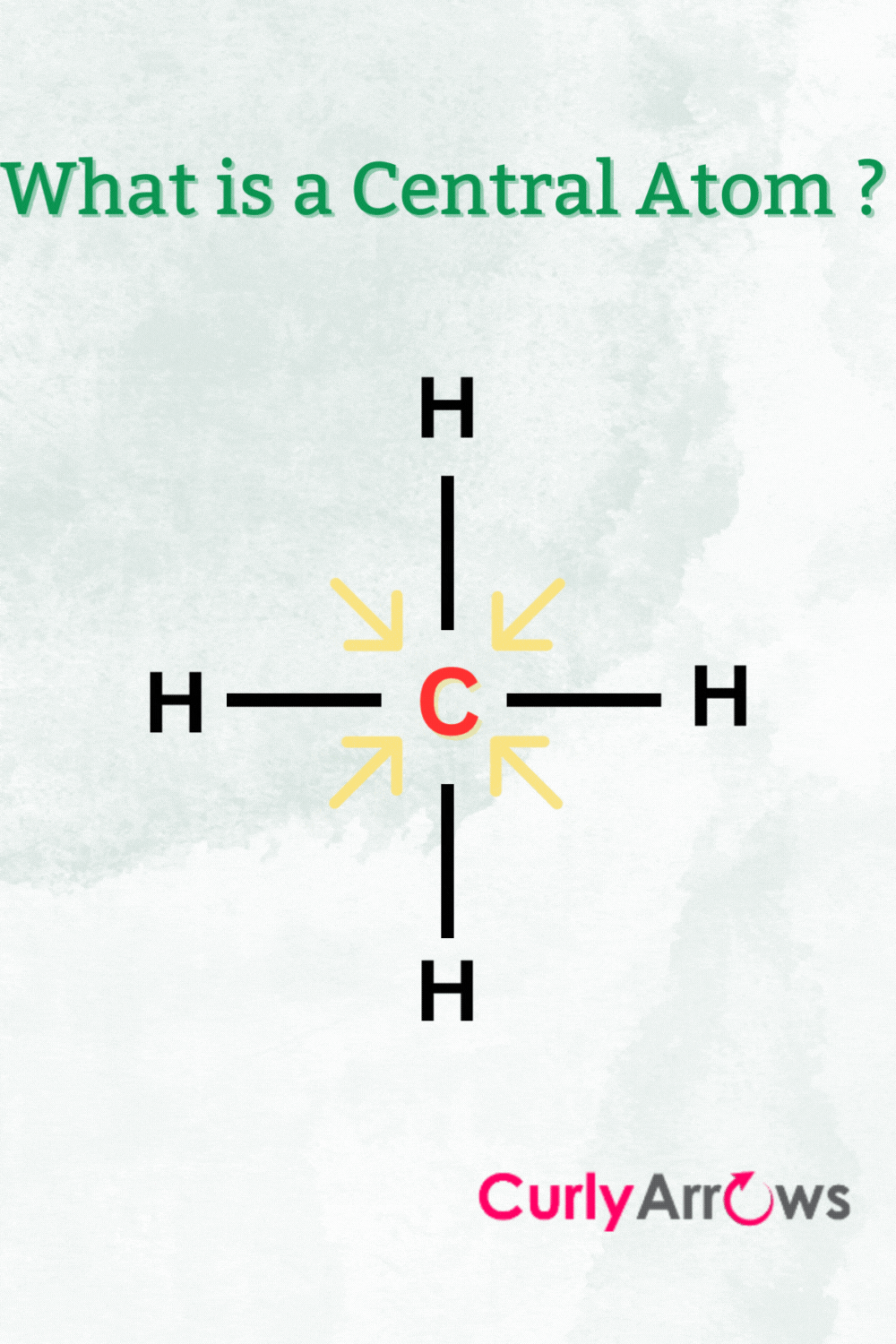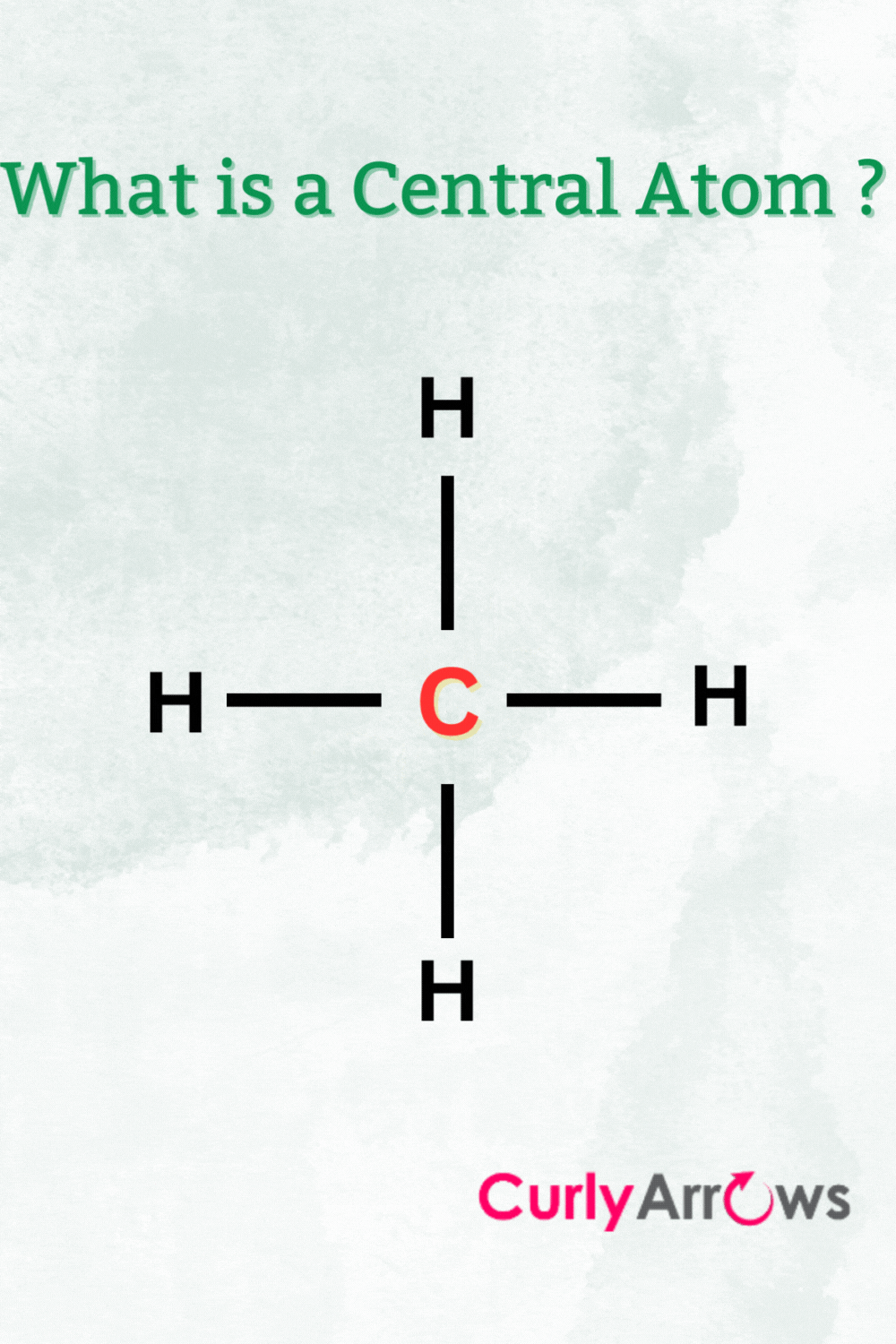
The molecular landscape is an intricate tapestry woven from various atoms; among these, determining the central atom, or the atom that serves as the backbone of a molecular structure, is paramount in understanding the chemical behavior and reactivity of a compound. The process of identifying the central atom is not merely a rote exercise but necessitates a comprehensive analysis of atomic characteristics, valency, geometry, and electronegativity. This article elucidates the methodological approach to discerning the central atom in a molecule.
Understanding Atom Valency
At the heart of any molecular structure lies the concept of valency, which refers to the ability of an atom to bond with other atoms. Valency is often dictated by the number of electrons in the outer shell of an atom, which influences how many bonds it can form. For instance, carbon, with a valency of four, frequently serves as a central atom due to its tetravalent nature, allowing it to forge stable covalent bonds with other elements. This propensity to form multiple bonds requires meticulous consideration, particularly in organic chemistry, where carbon frequently anchors molecular frameworks.
In contrast, atoms like hydrogen can typically bond only once, which limits their potential to assume a central role in complex molecules but makes them valuable for completing the structures emanating from a central atom. As one examines potential candidates for the central atom, environmental factors such as the local electronic configuration become critically important.
Electronegativity and Atomic Size
Electronegativity—an atom’s ability to attract electrons within a chemical bond—contributes significantly to ascertaining the central atom. Elements with higher electronegativity tend to draw electrons closer, which can stabilize particular molecular geometries. Consequently, when assessing molecules containing multiple atoms, the central atom may often be one with intermediate electronegativity, as this balance allows for the effective sharing of electrons among surrounding atoms.
Moreover, atomic size plays a crucial role in determining the central atom. Larger atoms are generally less effective at forming multiple bonds due to spatial constraints, while smaller atoms may be more suited for central positions because their compact nature allows for efficient orbital overlap. In structures such as hydrocarbons, carbon’s moderate size paired with its tetravalency overwhelmingly encourages its role as a ubiquitous central atom.
Analyzing Molecular Geometry
The spatial arrangement of atoms around a central atom is governed by the principles of molecular geometry, which dictates the shape of the entire molecule. Utilizing VSEPR (Valence Shell Electron Pair Repulsion) theory enhances our understanding of this geometric orientation. For example, a central atom bonded to four other atoms will adopt a tetrahedral configuration, a scenario that is typical for carbon in saturated hydrocarbons.
In other instances, the existence of lone pairs of electrons on the central atom significantly alters the geometry by exerting repulsive forces on the bonding pairs of electrons. For instance, water (H₂O) features an oxygen atom as a central atom in a bent geometry, unlike methane (CH₄), which exhibits a perfect tetrahedral configuration. Being adept at recognizing these geometric patterns enhances one’s ability to correctly identify the central atom across a multitude of chemical contexts.
Functional Groups and Hybridization
In addition to geometric considerations, one must acknowledge the influence of functional groups when determining the central atom. Functional groups are specific groupings of atoms that impart characteristic properties and reactivity to the molecule. In organic compounds, functional groups often dictate the molecular behavior and help identify the central atom, especially in complex structures where multiple functional entities coexist.
Hybridization also emerges as a pivotal concept in recognizing central atoms. The mixing of atomic orbitals to form new hybrid orbitals can enhance the understanding of bonding patterns and predict molecular shapes. For instance, an sp³ hybridization found in carbon leads to tetrahedral organization, while sp² arrangement produces trigonal planar geometry. Comprehending these hybridization states elucidates why certain atoms are favored as central due to their bonding capabilities.
Conclusion: Synthesizing the Factors
The determination of the central atom in a molecule is a multifaceted endeavor that hinges on an amalgamation of valency, electronegativity, atomic size, molecular geometry, functional groups, and hybridization. A systematic approach that encompasses these dimensions not only clarifies the role of the central atom but also enriches the understanding of the chemical properties and reactivity of the entire molecular structure. As one delves deeper into the realms of molecular chemistry, the discernment of a central atom unveils the intricate interplay between structure and function—the cornerstone of chemical science.