Graphene, often heralded as a “wonder material,” exhibits exceptional electrical, thermal, and mechanical properties that have attracted extensive research and commercial interest. Understanding how graphene is synthesized from its parent material, graphite, reveals not only the intricacies of material science but also underscores the potential transformative applications of nanomaterials. This exploration will delve into the comprehensive processes involved in graphene production, highlighting various methodologies, the underlying principles of each technique, and the implications for future innovations.
Graphite, a form of carbon structured in layered planes, serves as the precursor for graphene. The captivating aspect of graphene lies in its atomic configuration; a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice provides remarkable characteristics, such as a theoretical strength more than 100 times that of steel and exceptional electrical conductivity. However, the transition from graphite to graphene is laden with challenges, necessitating meticulous techniques for exfoliation and synthesis.
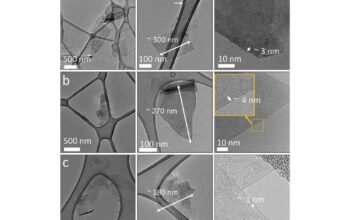
One of the most prevalent methods for producing graphene is mechanical exfoliation, often referred to as the “Scotch tape method” due to its simplicity. This technique involves physically peeling layers of graphene from bulk graphite using adhesive tape. The elegance of this approach lies in its ability to yield high-quality monolayers. However, while it is highly effective for laboratory purposes, scaling it for industrial production remains an obstacle due to inefficiency and the labor-intensive nature of the process.
In contrast to mechanical exfoliation, chemical exfoliation provides a more scalable avenue for graphene production. This method typically employs strong oxidizing agents to intercalate layers of graphite, promoting the separation of individual graphene sheets. Upon exfoliation, the resulting graphene oxide can be converted back to graphene through reduction processes. This transformation raises critical considerations regarding the preservation of electrical conductivity and structural integrity during reduction—factors essential for maintaining the desirable properties of the resultant graphene.
Another significant approach to synthesizing graphene is chemical vapor deposition (CVD). This technique involves the deposition of carbon-containing gases onto a substrate at elevated temperatures. The substrate, often metallic or ceramic, facilitates the growth of graphene layers as carbon atoms aggregate and crystallize. CVD is particularly renowned for producing high-quality, large-area graphene films, making it an attractive option for electronic applications and functional devices. However, the requirement of high temperatures and a controlled environment presents challenges concerning energy consumption and production costs.
Further amplification of graphene fabrication is achieved through liquid-phase exfoliation, a process that involves dispersing graphite in a solvent and subjecting it to ultrasonic waves. The mechanical agitation encourages the cleavage of graphite into graphene sheets. This method facilitates the production of graphene in liquid form, permitting easier integration into various applications, including composites and coatings. While the liquid-phase exfoliation technique offers a capacious production route, it often results in less-organized structures and varying sizes, necessitating further purification steps for critical applications.
As the realm of graphene synthesis expands, emerging methods such as electrochemical exfoliation gain prominence, leveraging electrochemical reactions to exfoliate graphite in an aqueous medium. This technique enhances the economic viability of graphene production while potentially allowing for automation and higher yield rates. The utility of electrochemical exfoliation extends beyond mere fabrication; it can also be adapted for the creation of graphene-based sensors and energy storage devices, thereby augmenting the versatility of graphene in the evolving landscape of nanotechnology.
Exploring these diverse synthesis techniques unveils the undercurrent of fascination surrounding graphene. The transitional journey from graphite to graphene encapsulates the broader narrative of material science—emphasizing innovation, painstaking processes, and interdisciplinary collaboration. Moreover, the unique properties of graphene stimulate an overarching curiosity about its applications, spanning fields such as electronics, energy, biomedicine, and structural materials.
Despite the myriad of methods available, challenges persist in terms of scalability, cost, and quality control. Achieving uniformity and reproducibility in the production of graphene remains at the forefront of research efforts. Additional studies interrogate the feasibility of integrating graphene into composites or hybrids with other materials, aiming to potentially optimize performance metrics while alleviating production hurdles.
The implications of graphene’s production method extend beyond scientific curiosity; they intertwine with the fabric of future technological advancements. As innovations in synthesis continue to evolve, the commercial viability of graphene depends on the establishment of economically sustainable production methods. The challenge lies in striking a balance between achieving high performance and managing costs.
In summary, the transformation of graphite into graphene encompasses a spectrum of methodologies, each driven by unique principles and implications. From mechanical peeling techniques to ambitious chemical approaches, the quest for efficient and scalable production remains an area ripe with opportunity. As the scientific community continues to unravel the potential of this remarkable material, the journey of graphene production from graphite stands as a testament to the relentless pursuit of knowledge and innovation in the world of materials science.