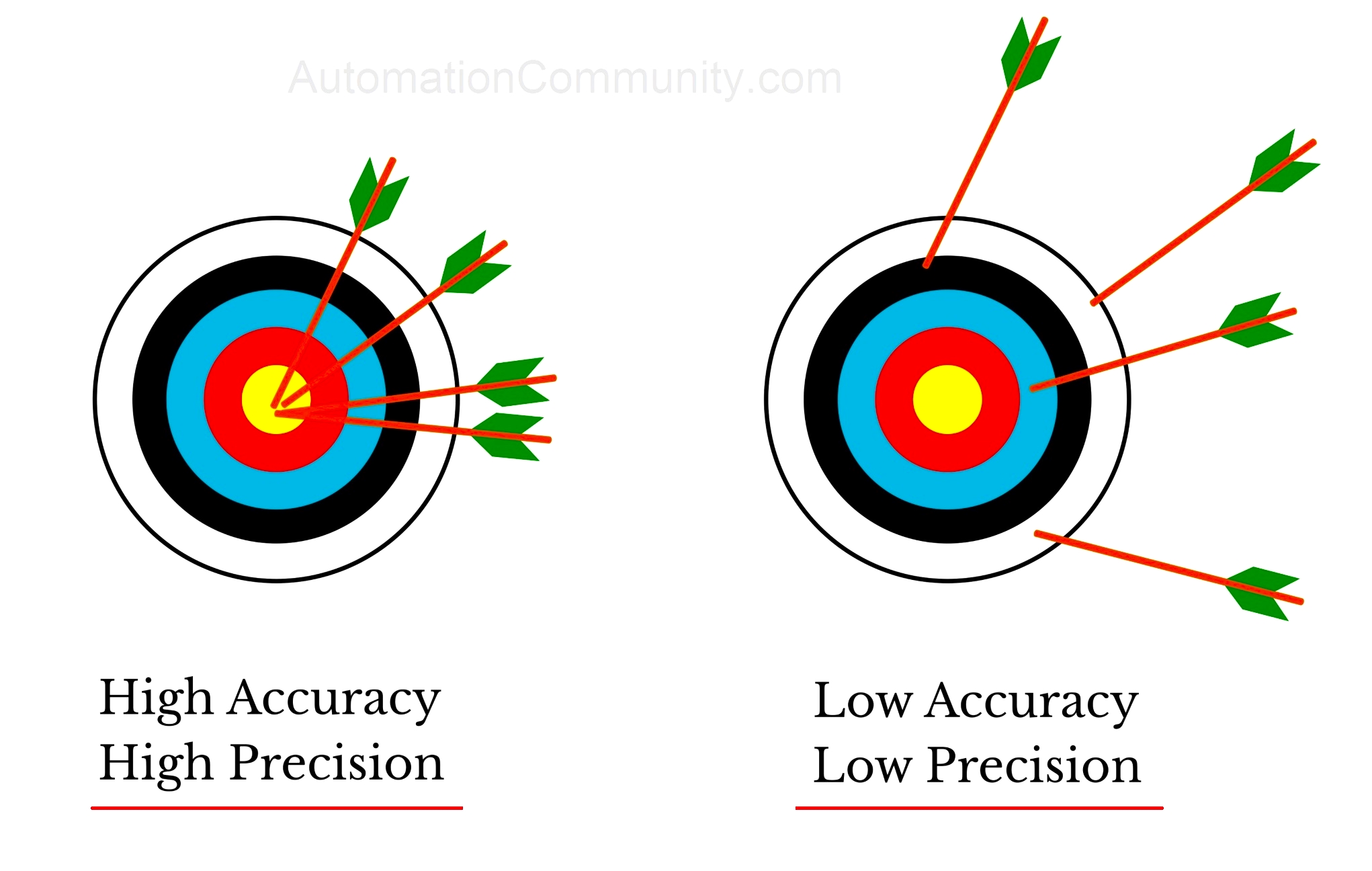
Argon, a noble gas with atomic number 18, has historically been overshadowed by its more reactive counterparts in the periodic table. Nonetheless, its unique properties, particularly in terms of accuracy and precision in various scientific applications, have garnered increasing attention. This article delves into the multifaceted relationship between argon’s inherent characteristics and its role in achieving precision at the atomic scale. Following a detailed exploration of argon’s physical and chemical properties, we will investigate its applications in various fields of research, shedding light on the implications of accuracy and precision in experimental science.
The atom of argon exists mostly in a gaseous state under standard temperature and pressure. This inertness, stemming from its filled outer electron shell, gives argon a distinctive stability that allows for minimal interaction with other elements. The implications of this inertness can be substantial across scientific disciplines, particularly in reducing the potential for contamination in controlled environments. This characteristic positions argon as a pivotal medium in fields such as spectroscopy, where its role as a noble gas can mitigate background interference, thereby enhancing measurement accuracy. Researchers utilizing argon as a carrier gas can achieve significantly more precise readings through spectral analysis, which is crucial in chemical identification and quantification.
The accuracy of gas measurements in various experimental apparatuses often hinges upon the control of environmental variables. Laboratory environments that employ argon-filled atmospheres are able to inhibit oxidation and other chemical reactions, thereby allowing for exceptionally precise thermal and photophysical measurements. The consistency of argon’s properties, including its specific heat capacity and thermal conductivity, contributes to its reliability as a standard reference gas. Such standards are vital when establishing fundamental scientific benchmarks, particularly in calibrating equipment used in quantitative analysis.
In the realm of atomic physics, precision becomes even more paramount. The use of argon in atomic beam experiments illustrates its instrumental role in advancing the boundaries of knowledge regarding atomic interactions and behaviors. Through techniques such as laser cooling and trapping, argon atoms can be studied with unprecedented accuracy. These methodologies require an environment where argon serves as a cooling medium, allowing researchers to observe atomic phenomena with minimal thermal disturbances. As a result, phenomena such as quantum oscillations can be measured with a level of precision that opens new avenues for research and understanding at the atomic scale.
Moreover, argon’s role is not limited to gas phase experiments. In the domain of material science, argon plasma etching stands out as a technique that provides high precision during the fabrication of micro-electronic devices. The use of argon in plasma generation forms a crucial part of processes aimed at defining nanoscale structures on semiconductor surfaces. The ability of argon to sustain a non-reactive plasma environment ensures that the etching process is both precise and repeatable, leading to improved device performance and reduced defects. This showcases how argon’s physical properties can enhance precision in practical applications, where the accuracy of dimensions at the nanoscale is critical.
An intriguing aspect of argon lies in its isotopic composition, particularly the presence of stable isotopes such as argon-36, argon-38, and argon-40. Isotopic analysis employing mass spectrometry leverages the accurate measurement of these isotopes to provide insights into geological and climatic shifts over geological time scales. The isotopic ratios of argon can reveal information about the age of rocks and the history of the Earth’s atmosphere. Such applications underscore the importance of precision in measuring isotopic abundances, which requires meticulous calibration against known standards to ensure accuracy in dating and environmental assessments.
As we delve deeper into the implications of argon’s properties for accuracy and precision, it is essential to consider future directions of research. The quest for achieving even higher levels of precision in measurements has led to advancements in technology, particularly in quantum mechanics and nanotechnology. Innovations in the use of argon can facilitate studies into quantum states and entanglement, paving the way for revolutionary applications in quantum computing and quantum cryptography. The interplay between argon and cutting-edge technologies paints a vibrant picture of the future of scientific research.
Industry applications of precision measurement with argon also deserve mention. In the pharmaceutical sector, for instance, argon is utilized to create inert environments for drug production, ensuring that products are not compromised by atmospheric contaminants. The inherent qualities of argon help maintain the integrity of sensitive compounds, demonstrating how precision in the pharmaceutical manufacturing process is fundamentally linked to the meticulous behavior of argon as a carrier gas. Such practical benefits extend to various industries where precision in chemical formulations remains crucial.
In summation, argon exemplifies a remarkable intersection of accuracy and precision within scientific disciplines ranging from atomic physics to material science. Its unique properties facilitate nuanced measurements that push the boundaries of our understanding of the atomic and molecular worlds. As research progresses, the role of argon is likely to expand further, continuing to challenge our concepts of measurement and control in scientific inquiry. This noble gas, often unnoticed in scientific discussions, holds a revered position as a critical agent in the pursuit of higher accuracy and precision at the atomic edge.