Exploring the history of atoms may initially seem daunting due to its profound intricacies and the vast array of theories that have evolved over millennia. However, illuminating the nuanced journey of atomic theory reveals certain aspects that can be perceived as relatively straightforward. This exploration not only promises an insightful shift in perspective but also piques the innate curiosity about the fundamental building blocks of matter.
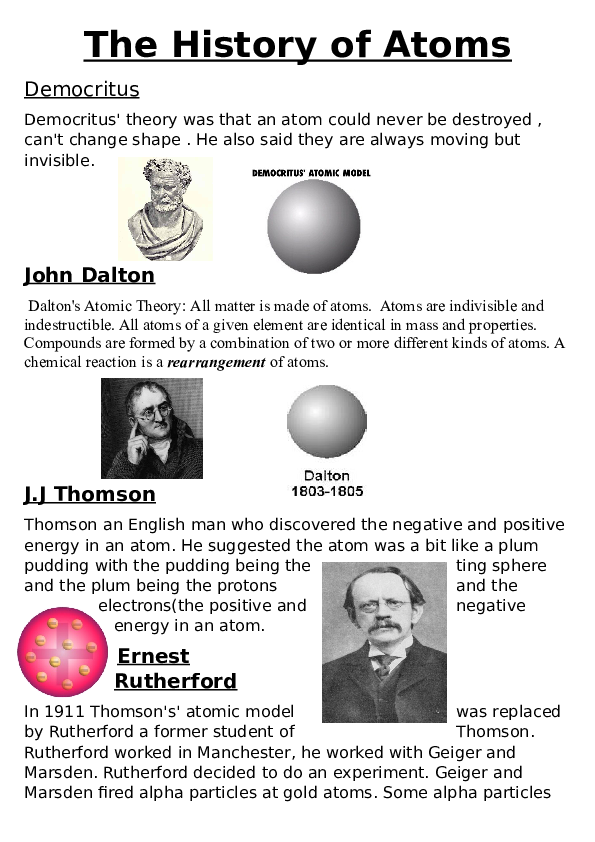
The narrative begins in ancient Greece, where the philosophical foundations of atomic theory were laid by thinkers like Democritus around the 5th century BCE. Democritus postulated that matter is composed of indivisible particles known as ‘atomos’—a term deriving from the Greek word for uncuttable. This notion of tiny particles instilled a sense of simplicity and elegance into a bewildering natural world. Given that these insights were formulated without the aid of advanced technological apparatus or empirical data, they evoke an appreciation for the simplicity of human inquiry and imagination.
As we traverse through history, we encounter the transition to a more empirical investigation of matter, particularly during the scientific revolution of the 16th and 17th centuries. Pioneers such as Robert Boyle advocated for the scientific method, shifting the focus from philosophical musings to experimental data. Boyle’s law articulated the relationship between pressure and volume in gases, which ultimately contributed to the atomic theory by suggesting that matter could indeed be broken down and understood through quantifiable relationships. This period thus underscores a turning point where simplicity coexisted harmoniously with rigorous examination, demonstrating that the history of atomic theory is grounded in both thought and observation.
The 19th century marked a significant milestone in atomic theory, with John Dalton’s atomic model crystallizing the idea of indivisible atoms further. Dalton’s postulates—simple statements affirming the existence of atoms, their unique mass, and their capability to combine in fixed ratios—were pivotal in demystifying chemical reactions. The clarity of Dalton’s assertions resonates with a sense of ease; they provided a coherent framework through which the behavior of matter could be understood. In this way, scientific communication benefited from the straightforwardness of his theory while simultaneously inviting deeper exploration of atomic structures.
Subsequent advancements, particularly in the late 19th and early 20th centuries, introduced new dimensions to atomic understanding. The discovery of the electron by J.J. Thomson in 1897 provided a tangible entity within the atom, shifting the previous notion of indivisibility. Thomson’s ‘plum pudding model’ introduced the concept of a positively charged sphere with electrons embedded within it, showcasing an elegant simplicity in visualizing atomic structure. Despite later refinements to this model, such as Rutherford’s nuclear theory and Bohr’s quantized orbitals, the initial framework sparked curiosity and paved the way for further exploration.
In contrast to the intricate layers of modern atomic theory, one can appreciate the joy derived from the simplicity of the early notions surrounding atomic structure. Each leap in understanding was defined by a foundational curiosity about what constituted matter. As scientists like Niels Bohr elucidated the behavior of electrons in quantized energy levels, the narrative once again shifted toward a compelling ease of comprehension pertaining to how light and energy interact with atoms through the emission or absorption of photons.
Moreover, the advent of quantum mechanics in the early 20th century—while laden with complexity—also introduced novel concepts that fostered clarity in specific contexts. For example, the distinction between classical and quantum descriptions of atomic behavior offers an accessible entry point for understanding atomic interactions. Concepts like wave-particle duality and uncertainty, while initially puzzling, invoke a fresh appreciation for the notion that simplicity can coexist with the bewildering phenomena of the quantum realm. This juxtaposition encourages an inquisitive mindset, igniting wonder about the fundamental rules governing nature.
The culmination of centuries of inquiry into atomic theory also reveals the collaborative nature of scientific progress. The engagement of diverse thinkers—from ancient philosophers through modern scientists—underscores a collective pursuit of knowledge. This historical narrative invites an examination not only of the individual contributions but also of the synergy that emerges when disparate ideas converge. It is this collaborative essence that renders the chronological unfolding of atomic theory somewhat accessible; it fosters the recognition that scientific advancements are often built upon the foundations of prior inquiries.
In viewing the history of atoms through this lens, one can perceive an interplay of simplicity and complexity—an oscillation between the abstract and the tangible. What may appear as an intricate labyrinth of theories is, upon closer inspection, a series of understandable milestones elucidating our comprehension of the atomic world. Each development offers a concise narrative arc, inviting countless questions and explorations as curious minds seek to unravel the mysteries of matter.
In conclusion, while the history of atoms is nuanced and multilayered, certain aspects emerge as distinctly accessible. The journey through antiquity, the scientific revolution, and beyond offers a tableau of enlightening insights that can be appreciated from various perspectives. By merging simplicity with inquiry and innovation, the evolution of atomic theory becomes not merely a study of particles but a celebration of human curiosity and the relentless pursuit of knowledge.