Neutrons are captivating subatomic particles that play a crucial role in the fabric of atomic structure and nuclear physics. But have you ever pondered the implications of a particle that possesses no net electric charge? This poses a playful question about the neutral nature of these entities. What challenges arise from their neutrality, and how does it affect their interactions? In this exploration of neutrons, we will navigate through their definition, properties, significance in atomic structure, role in nuclear reactions, and advancements in scientific research.
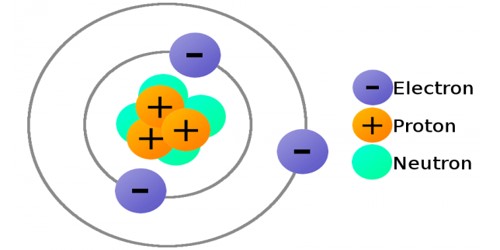
To commence, neutrons are one of the three primary constituents of an atom, alongside protons and electrons. Within the nucleus, neutrons coexist with protons, collectively referred to as nucleons. The presence of neutrons stabilizes the atomic structure, exerting a strong force that mitigates the electrostatic repulsion between positively charged protons. This strong nuclear force is essential for binding the nucleus together, overcoming the repulsive forces that arise from the like charges of protons.
The concept of neutron discovery traces back to the early 20th century, when physicist James Chadwick first identified this elusive particle in 1932. His groundbreaking work facilitated significant advancements in the field of nuclear physics, leading to the realization that neutrons exist alongside protons in the nuclei of atoms. Unlike protons, which carry a positive charge, neutrons possess a neutral charge, making them susceptible to the powerful forces that govern nuclear interactions.
Neutrons themselves are classified as baryons, a category of particles composed of three quarks. A neutron consists of one ‘up’ quark and two ‘down’ quarks, bound together by the exchange of gluons. This intricate assembly contributes to the neutron’s overall mass, which is notably larger than that of the electron but only slightly less than that of the proton. This negligible variance in mass plays a pivotal role in defining isotopes, variations of elements that contain the same number of protons but differ in neutron count.
Understanding the role of neutrons extends beyond atomic structure; they are fundamental to nuclear reactions and the processes that fuel the stars. In nuclear fission, the splitting of an atomic nucleus releases an immense amount of energy, largely driven by the behavior of neutrons. A neutron colliding with a heavy nucleus, such as uranium-235 or plutonium-239, can instigate a fission reaction, leading to the release of additional neutrons. This self-sustaining chain reaction not only powers nuclear reactors but also underpins the devastating force of nuclear weapons.
Moreover, neutron interactions are key to understanding the stability of atomic nuclei. For instance, elements exhibit multiple isotopes, some of which are stable while others are radioactive. Neutron-to-proton ratios are indicative of an element’s stability; too few or too many neutrons can result in an unstable configuration, leading to radioactive decay. In this context, neutron-rich isotopes can undergo beta decay, a process in which a neutron is transformed into a proton while emitting a beta particle and an antineutrino. This phenomenon is essential for the study of radiometric dating and the understanding of stellar nucleosynthesis.
The significance of neutrons in modern scientific research cannot be overstated. Neutron scattering techniques, for instance, have become invaluable tools in material science, allowing researchers to probe the atomic structure of various substances. By analyzing the way neutrons scatter when directed at a material, scientists can glean insights into atomic arrangement, molecular dynamics, and even phase transitions. These methods have applications that span a wide range of fields, including biology, chemistry, and condensed matter physics.
Despite our growing understanding, several challenges remain in the study of neutrons. The very nature that makes them neutral also complicates their detection. Traditional electromagnetic methods used to characterize charged particles are ineffective for neutrons, necessitating the development of specialized equipment. This challenge invites ongoing innovation in neutron detection technologies, which may provide even deeper insights into complex atomic phenomena.
Additionally, the quest for a comprehensive theoretical framework that unites quantum mechanics with gravity—a longstanding challenge within the field of physics—often brushes against the enigmas presented by neutrons and their interactions. How do these neutral particles fit into the grand tapestry of particle physics? What might their behavior reveal about the universe’s fundamental forces? These questions underscore the intrigue that surrounds the neutron, beckoning physicists to persist in exploration.
The story of neutrons invites us to appreciate the complexity of atomic architecture and the nuclear forces that govern it. From their discovery to their pivotal role in the cosmos, neutrons challenge our understanding and spur inquiry. As research progresses, these neutral particles may continue to hold the keys to unlocking new realms of knowledge, influencing everything from energy production to the very foundations of matter itself. In embarking on this intellectual journey, one is reminded of the delicate balances and intricate relationships that define our universe, where even a particle without charge can inspire curiosity and foster scientific advancement.