Color is an omnipresent facet of our sensory experience, impacting everything from art and design to signaling danger in nature. Our fascination with color in the physical realm arises not merely from its aesthetic qualities, but also from the intricate scientific principles that underpin it. The connection between atomic structure and coloration presents a compelling narrative in the realm of physical chemistry, revealing the often-invisible forces at play within atoms and molecules that govern the spectral properties of substances.
At the heart of color perception lies the interaction between electromagnetic radiation and matter. Visible light, which occupies a narrow band within the electromagnetic spectrum, comprises wavelengths ranging from approximately 400 to 700 nanometers. Different wavelengths correspond to different colors; for example, shorter wavelengths yield violet hues while longer wavelengths produce red. The atomic or molecular structure of a substance dictates which wavelengths are absorbed and which are reflected or transmitted, ultimately determining the color observed by the human eye.
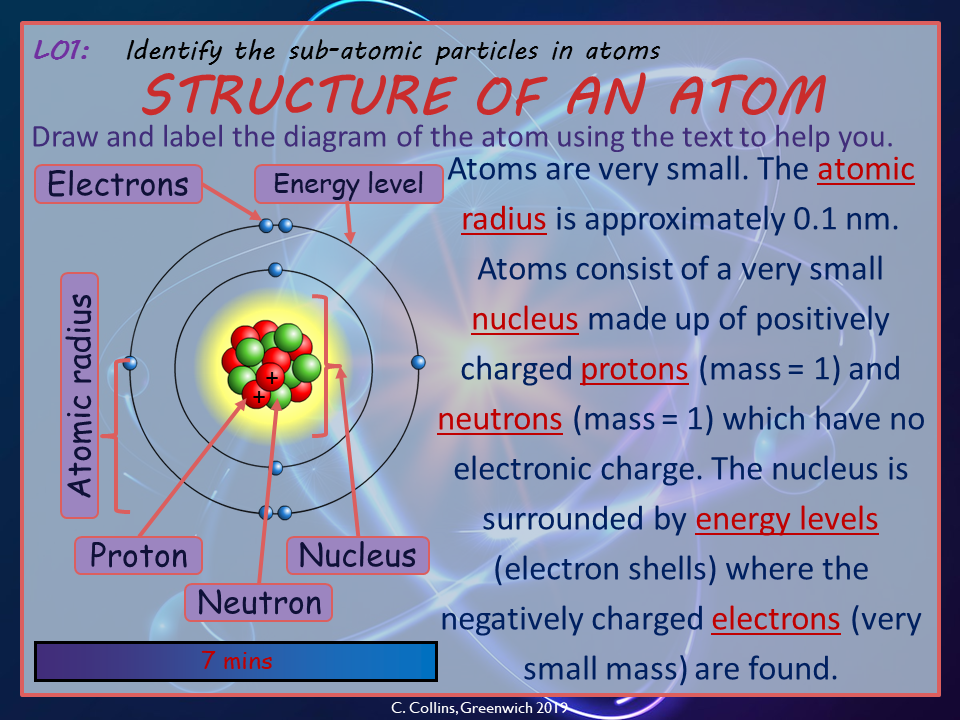
To understand this phenomenon, one must delve into the electronic structure of atoms. Each atom consists of a nucleus surrounded by electrons, which occupy distinct orbital configurations characterized by quantized energy levels. These energy levels dictate how electrons may transition between states, particularly when subjected to the influence of external energy sources, such as heat or light. When an electron in an atom absorbs energy, it may become excited, residing in a higher energy state. If the energy input is appropriate, the atom may subsequently release energy in the form of light as the electron returns to its ground state. This process is referred to as emission, and it is foundational in explaining color production.
The various colors observed in materials stem from their unique electronic configurations. Transition metals, for instance, exhibit vibrant colors due to the presence of partially filled d-orbitals. When light interacts with these metals, specific wavelengths are absorbed while others are transmitted or reflected, resulting in a distinct coloration. This selective absorption is predicated on the electronic transitions of the d-electrons, which facilitate the absorption of particular wavelengths, leading to characteristic hues that define numerous compounds.
In addition to transition metals, organic molecules with conjugated π-systems also demonstrate how atomic structure influences color. Organic dyes and pigments often feature alternating single and double bonds that allow for delocalized electrons. When such molecules are exposed to light, the absorption spectrum shifts; certain wavelengths are absorbed, causing others to manifest as observable color. The extent of conjugation plays a pivotal role in determining the color produced; as the π-system expands, colors can shift from blue to red, illustrating a direct correlation between molecular configuration and spectroscopic properties.
The crystal field theory offers an additional lens through which one can examine the interplay of atomic structure and color. According to this theory, the arrangement of ligands around a transition metal ion in a complex alters the energy levels of the d-orbitals, leading to varying light absorption patterns. These alterations result in specific colors in compounds such as potassium dichromate or copper sulfate. The geometrical arrangement of ligands creates splitting in d-orbital energy levels, leading to selective absorption of certain wavelengths. Consequently, the color of a transition metal complex is intricately tied to its ligand field environment.
Moreover, colorimetry and the study of color perception reveal how atomic structure influences not only the inherent color of a substance, but also the perception of color by the human eye. Human color vision is predicated on the optical properties of cone cells in the retina, which respond selectively to varying wavelengths of light. The combination of wavelengths absorbed by specific pigments within these cells leads to the brain’s interpretation of color. Thus, the interplay between atomic structure and the molecular composition of a substance not only dictates its physical appearance but also exerts a remarkable influence on human perception.
The phenomenon of fluorescence offers an astonishing illustration of how atomic structure can produce vivid colors through atomic interactions. Fluorescent materials absorb photons of one wavelength and re-emit them at a longer wavelength, creating intense colors. This extraordinary effect can be traced back to the electronic transitions within molecules that occur as they absorb energy. The structure of the molecules involved determines the efficiency of these transitions, making fluorescence not only a delightful visual experience but also a function of atomic physics.
In biological systems, the effects of atomic structure on coloration extend into the realm of biochemistry. The striking colors of various organisms, from peacock feathers to the vibrant hues of coral reefs, can be attributed to the unique pigments and their molecular arrangements. Carotenoids and chlorophyll, crucial for photosynthesis in plants, exhibit specific absorption characteristics based on their atomic structure. These pigments serve both protective and functional roles in nature, underscoring the profound relationship between atomic configuration and ecological dynamics.
In conclusion, the relationship between atomic structure and color provides a rich tapestry of inquiry within physical chemistry. From the fundamental interactions between electrons and photons to the complex arrangements of ligands influencing emission spectra, atomic structure acts as a crucial determinant of color in various materials. As one ponders the palette of colors that adorn the world around, the realization dawns that beneath the surface, intricate atomic mechanisms govern the seemingly simple phenomena observed through our eyes. This convergence of art, science, and perceptions invites continued exploration into the marvelous world of color and the fundamental principles that shape it.