Atomic hydrogen, a fundamental component of the universe, raises intriguing questions about the very fabric of matter. What is the essence of this simplest of atoms, and why is it so significant in the broader context of physics and chemistry? Exploring atomic hydrogen invites us to grapple with its formation, properties, and implications for both theoretical and applied sciences.
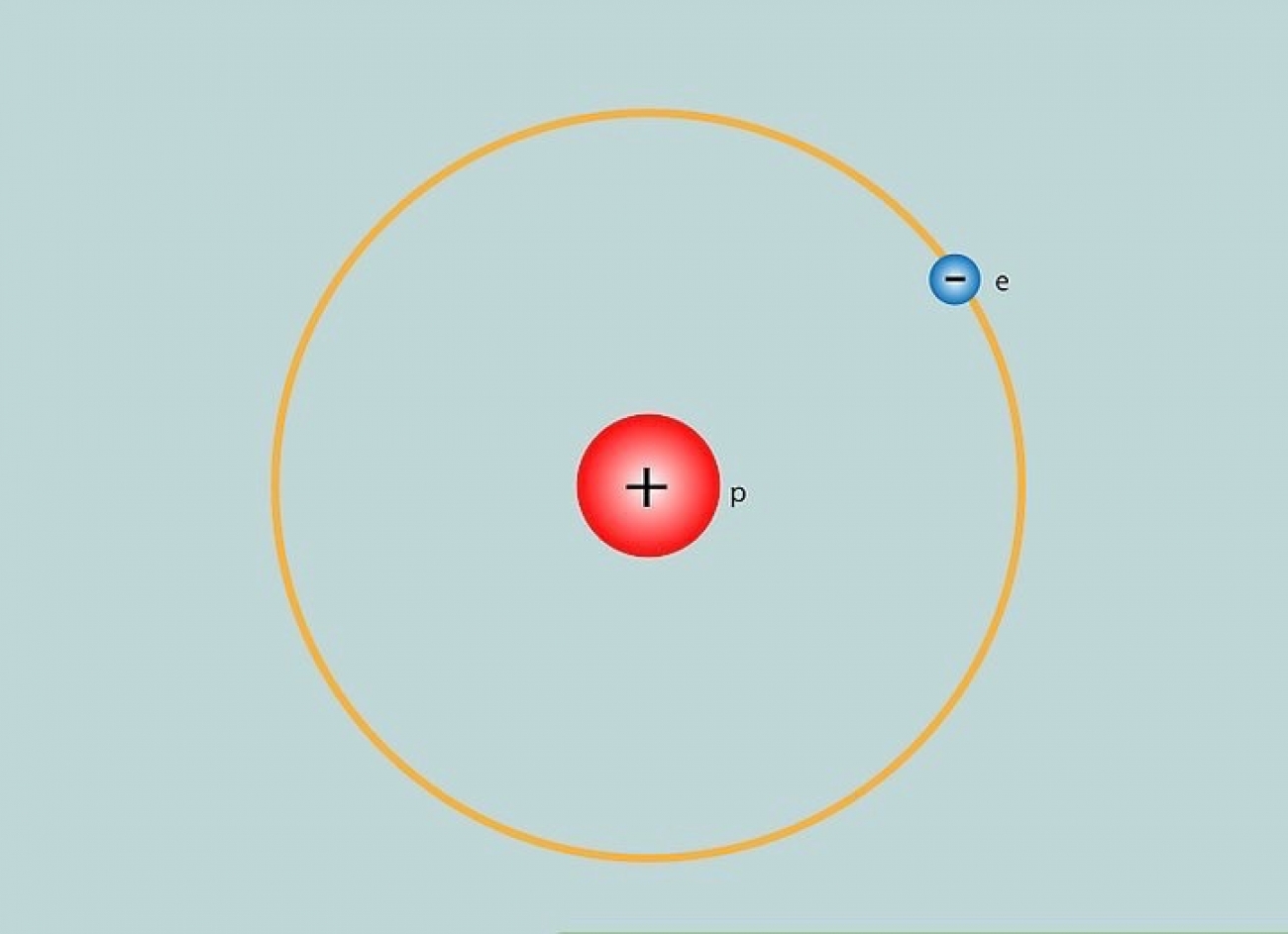
To commence, let us clarify the concept of atomic hydrogen. At its core, atomic hydrogen refers to a hydrogen atom that consists of a single proton and a single electron. This binary configuration is emblematic of the simplest element on the periodic table, with an atomic number of one. While the description may appear deceptively straightforward, the intricacies underlying atomic hydrogen are far more profound. When we contemplate hydrogen, we are not merely considering a chemical element; we are engaging with a pivotal player in the cosmic narrative.
The genesis of atomic hydrogen can be traced back to the primordial nucleosynthesis that took place shortly after the Big Bang. During this epoch, protons and neutrons coalesced to form the nuclei of the lightest elements, primarily hydrogen and helium. The subsequent period of recombination permitted electrons to amalgamate with these nuclei, giving rise to neutral atomic hydrogen. Thus, it is estimated that hydrogen constitutes approximately 75% of the baryonic mass in the universe, making it the archetypal building block of matter.
So, what properties imbue atomic hydrogen with such profound significance? One of its most notable characteristics is its propensity for reactivity. Hydrogen atoms can readily form bonds with other elements, facilitating the synthesis of a plethora of compounds—most prominently with oxygen to create water. Furthermore, atomic hydrogen manifests in various phases and states, including molecular hydrogen (H₂), which underpins many chemical reactions and processes essential for life.
This brings us to the curious realm of atomic hydrogen in isolation. Unbound and present in a gaseous state, atomic hydrogen exhibits unique spectral characteristics, which scientists employ to probe the universe. The emission and absorption spectra of atomic hydrogen present distinct lines which serve as fingerprints, allowing for the identification of hydrogen in distant stellar environments. As such, atomic hydrogen enables astrophysicists to decode the composition and dynamics of galaxies, an endeavor that explores the farthest reaches of the cosmos.
Yet, atomic hydrogen is not merely an observer of cosmic phenomena; it also participates assiduously in various physical processes. For instance, it plays a crucial role in quantum mechanics through phenomena such as electron transitions. When an electron within a hydrogen atom absorbs energy, it can transition between different energy levels, leading to the emission of photons when it returns to its ground state. This quantum behavior underpins much of modern atomic theory and paves the way for a myriad of applications, from lasers to semiconductor technology.
However, the seemingly innocuous atomic hydrogen possesses intrinsic challenges that warrant contemplation. Consider the paradox of stability versus reactivity. Atomic hydrogen is inherently unstable, readily forming diatomic hydrogen or reacting with other elements. This contrast presents significant hurdles in controlling hydrogen in scientific applications, especially in fields such as hydrogen fuel technology. Amidst the growing emphasis on sustainable energy sources, harnessing atomic hydrogen poses both a tantalizing opportunity and a daunting challenge. How can we effectively utilize this versatile element while mitigating its volatility?
Moreover, the exploration of atomic hydrogen extends beyond its conventional applications. It has found utility in advanced scientific research, including the field of material science. For example, atomic hydrogen is employed in catalytic processes and surface modification. The ability to manipulate hydrogen interactions at the atomic level opens avenues for the development of novel materials with tailored properties, potentially revolutionizing industries ranging from electronics to pharmaceuticals.
In juxtaposing these facets of atomic hydrogen, it becomes evident that this single atom encapsulates a multitude of scientific narratives. From its primordial origins to contemporary applications, atomic hydrogen serves as a linchpin of knowledge in both theoretical physics and practical applications. However, the interplay between its simple composition and complex behaviors demands a deeper understanding of the atomic world.
As we engage with the concept of atomic hydrogen, we must also confront the broader implications of our understanding. For instance, how does the study of such a fundamental particle inform our comprehension of the universe? What does it reveal about the nature of matter and energy? Delving into these inquiries is not merely an academic pursuit; it prompts us to reconsider our place within the cosmos and the fundamental forces at play.
In conclusion, atomic hydrogen is far more than a mere constituent of matter; it symbolizes a bridge between the elementary and the complex. Through its myriad properties, challenges, and roles in scientific inquiry, atomic hydrogen embodies the dance of creation and transformation inherent in the universe. As we continue to explore and unlock the secrets of this fundamental atom, we inevitably deepen our understanding of the universe itself—an endeavor that is both playful in its inquiry and serious in its quest for knowledge.