Atomic spectra is a fascinating phenomenon that underscores the intricate relationship between matter and electromagnetic radiation. This intricate interplay is a fundamental tenet of spectroscopy and plays a pivotal role in our understanding of atomic and molecular structures. The study of atomic spectra allows scientists to glean invaluable insights into the energy levels, electronic configurations, and intrinsic properties of atoms and ions. This article will elucidate the diverse manifestations and classifications of atomic spectra, emphasizing their significance in various scientific disciplines.
To commence, it is essential to define atomic spectra. The term refers to the characteristic spectrum of electromagnetic radiation emitted or absorbed by atoms. When an atom interacts with energy—whether through heat, electricity, or electromagnetic waves—it can become excited, prompting its electrons to transition from lower energy levels to higher ones. Upon returning to their original states, these electrons emit photons of specific wavelengths, which constitute the atomic spectrum. This emitted light reveals a plethora of information about the atom, encapsulated in distinct lines, bands, or continua across the electromagnetic spectrum.
Atomic spectra can be broadly categorized into two primary types: emission spectra and absorption spectra. Emission spectra arise when electrons drop from higher energy states to lower ones, releasing energy in the form of light. Conversely, absorption spectra emerge when photons pass through a cooler gas or liquid. In this instance, certain wavelengths are absorbed to elevate electrons to higher energy states, resulting in characteristic dark lines against a continuous spectrum of light.
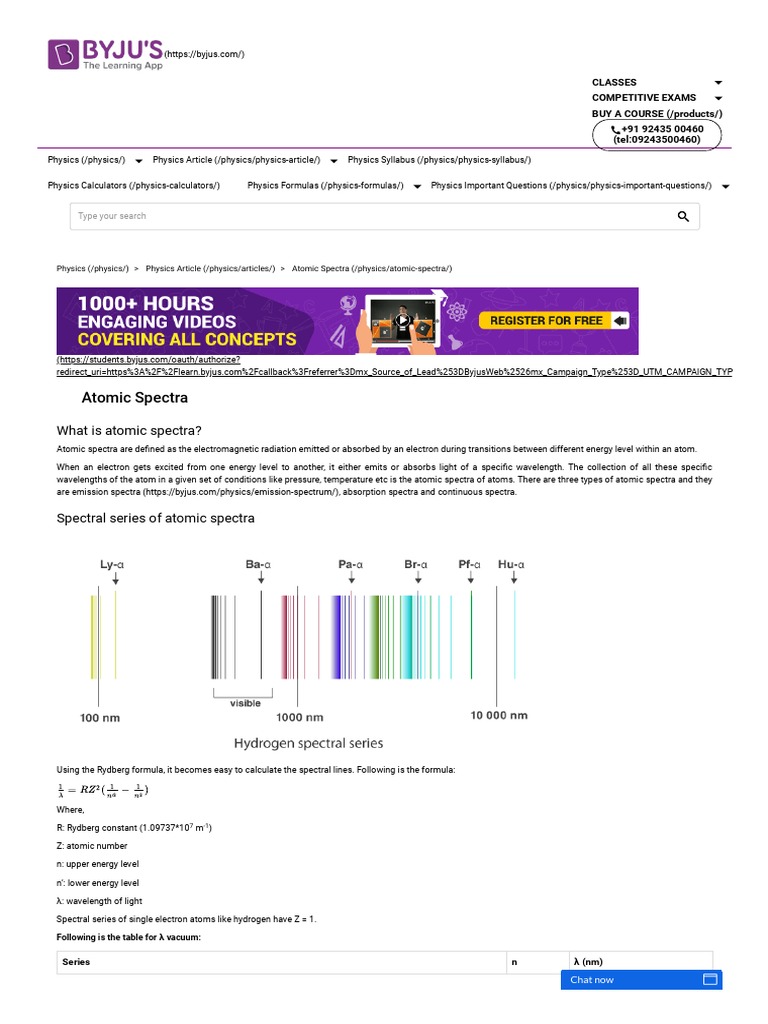
Emission spectra are further subdivided into continuous, line, and band spectra. Continuous emission spectra represent a seamless spectrum of light without any gaps, typically produced by incandescent solids or liquids. An example is the spectrum of a solid or liquid heated to incandescence, where the emitted light covers all visible wavelengths. The line spectrum, however, is comprised of sharp lines at specific wavelengths and is emblematic of gaseous substances heated to high temperatures. Hydrogen, for instance, exhibits a series of distinct spectral lines known as the Balmer series in the visible range, correlating to transitions between its energy levels.
On the other hand, band spectra arise from complex molecules and are characterized by groups of closely spaced lines. These spectra typically result from vibrational and rotational transitions within molecules, providing insights into molecular structures and interactions. Band spectra are particularly significant in the fields of molecular spectroscopy and astrophysics, where they can inform scientists about the composition of distant stars and galaxies.
Absorption spectra also exhibit unique features worth noting. The series of dark lines or bands corresponds to specific wavelengths where photons have been absorbed by electrons in atoms or molecules, facilitating transitions between energy levels. These distinct patterns are crucial for identifying elements in various samples, as they function akin to fingerprints, allowing for the precise determination of chemical compositions. For instance, when a star’s light traverses its surrounding gaseous atmosphere, elements such as hydrogen, helium, and heavier elements absorb specific wavelengths, producing a discernible absorption spectrum. Such spectral analysis has fed into our understanding of stellar compositions and the lifecycle of stars.
The applications of atomic spectra extend beyond traditional chemistry and physics. In astronomy, spectroscopic techniques have been instrumental in elucidating the chemical makeup of celestial bodies and phenomena. For example, the Doppler effect can modify the wavelengths of light emitted from stars, allowing astronomers to ascertain their motion—whether they are moving towards or away from the observer. This principle of redshift and blueshift has significantly advanced our comprehension of the expanding universe.
Furthermore, atomic spectra play an integral role in practical applications such as in the development of lasers, fluorescent lights, and gas discharge tubes. By manipulating the energy levels of electrons within specific atoms or molecules, scientists can generate coherent light, facilitating numerous technologies that permeate daily life. Understanding these spectra is crucial for designing more effective light sources and imaging systems.
Moreover, in the field of medicine, atomic spectra are harnessed in techniques such as spectroscopy for diagnostic imaging and analysis. The precise measurement of atomic transitions provides vital information about biochemical processes, enabling advancements in medical diagnostics and treatments. Similarly, the study of atomic spectra has applications in environmental science for monitoring pollutants and examining ecological processes. Analytical techniques like atomic absorption spectroscopy have revolutionized our capabilities to detect trace elements in environmental samples.
It is pivotal to recognize the historical context in which the study of atomic spectra has evolved. The field of spectroscopy can trace its origins to the late 19th and early 20th centuries, with foundational contributions from luminaries such as J.J. Balmer and Niels Bohr. The Bohr model of the atom, particularly, revolutionized our understanding by introducing quantized energy levels, elucidating electron transitions, and shaping modern quantum mechanics. Subsequent advancements in spectroscopy have enabled precise measurements and an extensive exploration of atomic and molecular behavior.
In summation, atomic spectra constitute a cornerstone of scientific inquiry, interspersing fundamental principles of physics and chemistry with vast applications across diverse fields. By studying the light emitted or absorbed by atoms, scientists unlock a treasure trove of knowledge about the universe’s building blocks, enhancing our comprehension of both atomic structure and larger cosmic phenomena. The continuous exploration of atomic spectra promises to yield further insights that will advance both theoretical frameworks and practical technologies in the years to come.