In the realm of physics and chemistry, the measurement of mass occupies a central position, especially when it pertains to the atomic scale. One might ponder: Why can mass, at its most fundamental level, not be measured directly? This inquiry unveils a plethora of complexities that not only challenge our typical understanding of mass but also invite a deeper exploration into the subatomic world.
To embark on this fascinating exploration, it is paramount to first delineate the nature of mass itself. Mass can be perceived in two principal contexts: the intrinsic mass of particles and the effective mass they exhibit when subjected to external forces. At the atomic level, particles such as protons, neutrons, and electrons come together to form atoms, yet measuring their mass is not as straightforward as one might assume.
The conventional means of measurement rely on weighing. In macroscopic terms, mass can be measured using balances that compare an object against a standard mass. However, as one ventures into the microscopic realm, difficulties arise. The very act of measurement can perturb the system being observed; thus, one must grapple with the Heisenberg Uncertainty Principle. This principle posits that certain pairs of physical properties, like position and momentum, cannot both be precisely measured simultaneously. Consequently, attempting to measure the mass of an atom inherently disturbs its state, leading to ambiguous results.
Furthermore, the challenges of measuring atomic mass transcend mere theoretical considerations. When isolating an atom to measure its mass, scientists typically rely on techniques that involve interaction with light or other particles. This interaction can alter the energy levels and, correspondingly, the effective mass. This leads to the intriguing question: Can we ever obtain a true value for atomic mass, or will we always be left with an approximation?
To elaborate, let us examine the concept of the atomic mass unit (amu). While the atomic mass unit provides a standard for quantifying the mass of atoms, it remains an average derived from isotopic distributions. Take, for example, carbon; the atomic mass of a carbon atom is approximately 12 amu. However, this value is contingent upon the isotopic composition of a sample. Carbon has several naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. Each of these isotopes harbors a distinct mass, thus complicating the quest for a definitive atomic mass. The question then arises: Is it possible to speak of an absolute mass for an atom, or is this notion inherently flawed?
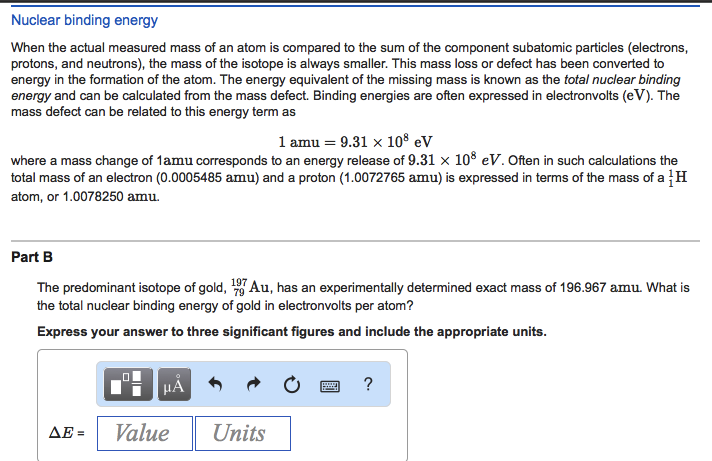
Moreover, let us delve into the role of binding energy in the context of mass measurement. When atoms combine to form isotopes, the binding energy—the energy required to disassemble a nucleus into its constituent protons and neutrons—contributes to changes in mass. According to Einstein’s mass-energy equivalence principle, mass can be converted to energy and vice versa. Hence, the mass of a nucleus can differ significantly from the sum of the masses of protons and neutrons due to the release of binding energy, further muddling our ability to measure mass at the atomic scale accurately.
To make matters even more intricate, we must consider the concept of virtual particles and quantum fluctuations. Quantum field theory posits that even a ‘vacuum’ is teeming with transient particles that momentarily pop into and out of existence. These virtual particles contribute to the effective mass of observable particles. What, then, can we conclude about the mass of an atom in this churning sea of fluctuations? How does one ascertain a mass in a reality that is inherently probabilistic?
This brings us to the nuanced distinction between mass and weight. In everyday experiences, weight is often conflated with mass. However, weight is a gravitational force exerted on an object and can change depending on the strength of the gravitational field. On the other hand, mass—the amount of matter in an object—remains invariant regardless of location. Thus, the quest to measure mass at the atomic scale must differentiate between these concepts, further complicating the measurement endeavor.
In addition to these theoretical challenges, technological limitations must also be acknowledged. The creation of instruments capable of measuring atomic masses with precision is a significant challenge. Mass spectrometry, for instance, is a sophisticated technique that separates ions according to their mass-to-charge ratio and offers insights into atomic composition. However, this process is indirect and reliant on several assumptions, including calibration and the ideal behavior of ions—further straining the notion of a definitive mass.
In summation, the conundrum of measuring atomic mass is multifaceted and riddled with challenges that span theoretical, experimental, and technological domains. From the perturbative nature of measurement and the implications of quantum mechanics to the intrinsic complexities of isotopic variations and binding energy considerations, it is evident that mass at the atomic level eludes straightforward quantification. As we probe deeper into this paradox, the interplay between mass, energy, and the very fabric of reality begs further exploration. Therefore, the question persists: Can we ever hope to grasp the true nature of mass at such a diminutive scale? Or will this remain a tantalizing question that forever dances just beyond our reach?