The realm of hardness encompasses an intriguing spectrum, where materials engage in a silent competition for supremacy. While diamonds reign supreme, possessing an unrivaled hardness due to their unique crystalline structure of carbon atoms, the quest for alternatives invokes a profound inquiry: what is the hardest non-metal material, capable of withstanding the rigors of time and pressure, aside from the gleaming gemstone? In this examination, we delve into the nuances of hardness, culminating in the exploration of boron carbide—the most formidable non-metal contender in this geological beauty pageant.
To examine the characteristics of hardness, one must delve into the Mohs scale—a rather venerable tool in the domain of mineralogy. This scale, conceived in 1822, ranks minerals based on their ability to scratch one another, thereby establishing a hierarchy that capably illustrates the relative tenacity of various substances. At the pinnacle of the scale, diamond stands tall at ten. In contrast, various materials occupy positions like graphite, talc, or quartz, each offering a unique testimony to their molecular composition and atomic arrangements.
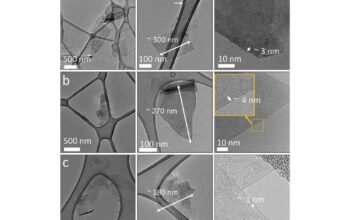
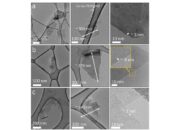
Beyond diamonds, our journey through the crystalline landscape leads us to boron carbide, an enigmatic compound poised elegantly at a hardness rating between 9.0 and 9.5 on the Mohs scale. Boron carbide, an astonishing configuration of boron and carbon atoms, is more than just a mere contender; it embodies the very spirit of resilience and tenacity. Its formula, B₄C, results in a robust network of covalent bonds, allowing it to withstand extreme conditions and grant it a remarkable ability to absorb shock. As we peel back the layers of this material, a portrait of its structure emerges: a complex, three-dimensional lattice of atoms, harmoniously interwoven into an intricate tapestry of strength.
To further elucidate the uniqueness of boron carbide, consider its synthesis. The production process often involves the reaction of boron trioxide with carbon, yielding a polycrystalline form endowed with extraordinary properties. This methodology not only paints a picture of its genesis but also highlights its practical applications, which span across various industries. From the manufacturing of ballistic armor to its use in abrasives, boron carbide exhibits utility matched only by its inherent capacity for hardness. The defense sector takes particular interest in boron carbide for its lightweight and exceptional hardness properties, making it ideal for armor plates in military vehicles.
Furthermore, boron carbide’s role in nuclear reactors adds another layer to its multifaceted identity, as it serves as a neutron absorber. Herein lies the beauty of science—the duality of a material whose hardness rivals diamonds while simultaneously playing a critical role in safety and security. Essentially, boron carbide is not merely a hard substance but a cornerstone in both engineering and defense, revealing the versatility of materials science.
In juxtaposition, we must acknowledge other notable contenders that have emerged in the discourse surrounding hardness. Silicon carbide (SiC), for instance, can proudly claim its place as a formidable substance. An allotrope of carbon similar to diamonds, it boasts a hardness approaching that of boron carbide, rating roughly between 9 and 9.5 on the Mohs scale. This compound radiates with various applications, particularly in the realms of electronics and high-temperature devices. Silicon carbide can handle the heat without a hint of fatigue, embodying both resilience and utility.
Moreover, transitioning to the ceramic sphere, alumina (Al₂O₃ or corundum) warrants consideration. Although ranking below boron carbide and silicon carbide at about 9 on the Mohs scale, its widespread availability and adaptability for industrial utilizations bolster its relevance. From abrasives to cutting tools, corundum displays admirable durability, a testament to the nuanced interplay between hardness and practicality in material selection.
To distill our exploration into a cohesive understanding, it becomes paramount to appreciate the poetic elegance of materials like boron carbide and their ilk. The visual imagery evoked by such compounds—their shimmering surfaces, resilient natures, and structural complexities—transcends the mundane to deliver a profound narrative within the fabric of science. From gemstones buried deep in the earth to synthesized components fostering innovation, the journey of hardness in non-metal materials is but an allegorical reflection of resilience in nature itself.
In conclusion, the quest for the hardest non-metal material reveals much about our universe’s intricate tapestry. Boron carbide emerges not merely as a rival to diamond in hardness, but as a multifaceted gem—durable, adaptable, and quite literally, a cornerstone of modern technology. Whether through its extreme resistance to abrasive wear or its invaluable applications in defense and industry, boron carbide stands testament to the wonders of molecular ingenuity. Its compelling narrative intertwines with the essence of scientific exploration, inviting us to marvel at the myriad forms of resilience etched across the planet’s mineral kingdom, each vying for their place in a world steeped in wonder and discovery.