Graphite, a crystalline form of carbon, has fascinated chemists and materials scientists for centuries. Most often recognized for its remarkable electrical conductivity and lubricating capabilities, it possesses an intriguing property: hardness. This quality may seem paradoxical, particularly when juxtaposed with its allotrope, diamond, which is far harder. To delve into why inorganic graphite is hard, we must explore its atomic structure, bonding characteristics, and the physical phenomena that govern its robustness.
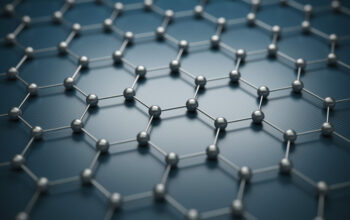
The hardness of any material is fundamentally dictated by its atomic structure and the nature of the interactions between its constituent atoms. Graphite comprises layers of carbon atoms arranged in a hexagonal lattice, forming two-dimensional sheets. This planar structure is a hallmark of graphite, and it is this arrangement that elevates its hardness to a degree that merits examination. One must consider the covalent bonds that exist within these layers versus the weaker van der Waals forces between the layers.
Within each sheet, each carbon atom is covalently bonded to three other carbon atoms, forming a strong, stable network. These covalent bonds are significantly strong due to the sp2 hybridization of the carbon atoms. This hybridization allows the unhybridized p-orbitals to overlap, delocalizing electrons across the layers and creating resonance. The result is a compound that exhibits substantial intrinsic hardness within the layers, as these bonds are exceedingly strong and resist deformation.
Despite its layered structure, the hardness of inorganic graphite cannot be understood solely through the lens of covalent bonding. The planar arrangement enables a high degree of alignment among the carbon atoms, contributing to the stability of the crystal lattice. When subjected to compressive forces, these structures resist shear due to the orientation of the layers, thereby showcasing the interplay between structural alignment and mechanical resistance. This characteristic leads to an enhanced hardness within the dimensions of the sheets, where dislocation is less favorable.
Another salient feature of graphite’s hardness is the absence of crystalline defects. In many materials, defects and dislocations can facilitate movement and deformation, reducing overall hardness. In purified forms of graphite, particularly those used in various industrial applications, the lack of significant structural imperfections elevates their hardness, rendering them impervious to certain types of mechanical stress. This pristine condition is often achieved through methods such as high-temperature graphitization, which allows for the reorganization of the carbon lattice into a more uniform structure.
The interplay of interlayer interactions in graphite also merits attention. While the van der Waals forces between the sheets are relatively weak compared to covalent bonds, they play a crucial role in maintaining structural integrity. Their cumulative effect resists shear failure, contributing to an overall hardness that can be surprising for such a seemingly layered material. Notably, these interlayer forces allow for a unique characteristic: while graphite is technically hard within its layers, it can also be plied and cleaved between them. This duality is what permits the industry to utilize graphite not just as a hard material but also as an effective lubricant.
Furthermore, the hardness of inorganic graphite is influenced by the method of its synthesis. Synthetic graphite, created through high-temperature processes such as the graphitization of polymer precursors, can exhibit hardness levels that rival those of natural graphites, if not surpassing them. The temperature and time parameters during synthesis dictate the degree of crystallinity achieved, impacting the hardness through enhanced structural integrity and fewer defects. This adaptability underscores the significance of controlled synthesis in tailoring the hardness of graphite for specific applications.
In addition to synthetic methods, the incorporation of other elements during the synthesis process can also modulate hardness. Graphite can be doped with various metals or non-metals to create composite materials that exhibit enhanced mechanical properties, including hardness. For instance, boron nitride and carbide additives can improve the inherent hardness of graphite, enabling its use in specialized applications where additional strength is paramount.
Moreover, the role of temperature cannot be overstated. The hardness of graphite is temperature-dependent; above certain thermal thresholds, the movement of atoms increases, allowing for a degree of plasticity that may diminish hardness. However, within a stable temperature range, graphite’s covalent framework remains robust, sustaining its hard nature. This nuanced relationship between temperature and hardness is vital for understanding how graphite performs under varying environmental conditions.
In conclusion, the hardness of inorganic graphite emerges from a complex interplay of atomic structure, bonding characteristics, synthesis methods, and interlayer interactions. Its semi-metallic conductivity and layered structure provide unique advantages, forming a material that is both robust and versatile. The duality of its hardness—being resilient within layers yet pliable across layers—opens avenues for exploring its application in nanotechnology and materials science. Each discovery propels further inquiry into its potential, inspiring scientists and researchers to harness its uniqueness in innovative ways.