John Dalton, an eminent figure in the annals of chemistry and physics, is predominantly celebrated for his groundbreaking atomic theory, which catalyzed a paradigm shift in the understanding of matter. This theory articulated a cohesive framework that allowed scientists to grasp the fundamental nature of atoms, thereby laying the groundwork for modern atomic physics. Dalton was not merely a scientist; he was a multifaceted individual—an educator, meteorologist, and philosopher. His relentless quests for knowledge and understanding of the natural world inspired generations of subsequent scientists.
Dalton was born on September 6, 1766, in Eaglesfield, England. He hailed from a modest Quaker family, where he was the youngest of six children. Dalton’s early forays into education involved the study of mathematics and the natural sciences, spurred by his insatiable curiosity. His academic journey was characterized by a firm commitment to empirical observation and rigorous experimentation. The inception of his illustrious career was undoubtedly influenced by the prevailing Enlightenment ideals that championed reason and scientific inquiry.
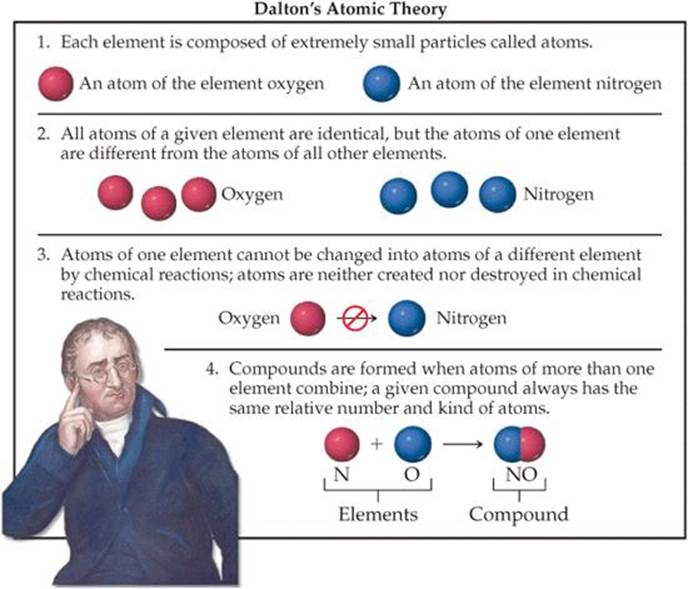
Throughout the early nineteenth century, the scientific community was ensnared in various theories about the composition of matter. However, it was Dalton’s atomic theory, articulated in the early 1800s, that provided one of the most cohesive explanations regarding the nature of substances. Dalton’s atomic theory comprises several pivotal postulates:
- All matter is composed of indivisible particles called atoms. Dalton proposed that atoms are the fundamental building blocks of matter. Unlike macroscopic objects, atoms are indestructible and cannot be subdivided into smaller units through chemical processes.
- All atoms of a given element are identical in mass and properties. This postulate suggests that atoms of the same element share common characteristics, which leads to the conclusion that they can participate in chemical reactions in predictable ways. For Dalton, each element is defined by the unique mass of its atoms.
- Compounds are formed by the combination of atoms of different elements. Dalton asserted that atoms of different elements can combine in specific ratios to form compounds. This introduced the concept that chemical reactions entail the rearrangement of atoms rather than their creation or destruction.
- Chemical reactions involve the reorganization of atoms. Dalton emphasized that during a chemical reaction, atoms are simply rearranged to form new substances. The atoms themselves remain unchanged, thereby reifying the notion of conservation of mass.
Dalton’s atomic theory was revolutionary in its simplicity and profundity. It addressed a fundamental observation: the diversity of matter can be explained through the combination and arrangement of a limited number of atoms. This insight captivated the scientific community and inspired subsequent investigations into the nature of atomic interactions. Moreover, Dalton’s insistence on the measurable and quantifiable properties of atoms aligned seamlessly with the burgeoning field of chemistry that was concerned with precision and reproducibility.
The allure of Dalton’s atomic theory extends beyond its scientific significance; it begs deeper inquiries about humanity’s intrinsic quest to understand reality. In an era marked by rapid industrial growth and technological innovation, Dalton’s theory provided an intellectual framework that reinforced the belief in a rational universe. It posited that the complexities of chemical behavior could ultimately be deciphered through a finite number of atomic interactions, further instilling a sense of order amidst the chaos of the natural world.
Dalton’s work inevitably encountered challenges and evolution. Subsequent scientists, notably J.J. Thomson and Ernest Rutherford, built upon and refined Dalton’s ideas as the quantum nature and subatomic structure of matter became evident. Thomson’s discovery of the electron in 1897 and Rutherford’s subsequent nuclear model of the atom in 1911 illuminated complexities within atomic structure that Dalton’s theory could not address. Nevertheless, Dalton’s foundational principles remain integral to the current understanding of atomic theory.
The historical significance of Dalton’s atomic theory cannot be overstated. It introduced the concept of the mole and led to the formulation of the law of multiple proportions, which solidified Dalton’s stature as a pioneer in stoichiometry. Furthermore, his meticulous observations and calculations laid the groundwork for a more systematic approach to chemistry, which encouraged future generations of chemists to embrace a quantitative methodology.
In an era where contemporary researchers continue to explore the intricacies of atomic and subatomic particles, it is vital to acknowledge Dalton’s larger legacy. He might not have envisioned the quantum mechanical complexities that govern today’s understanding of atoms. Still, his unwavering commitment to empirical inquiry and rational thought instilled a scientific rigor that has continued to reverberate through the corridors of physics and chemistry.
In conclusion, John Dalton’s contributions to atomic theory formed the cornerstone of modern chemistry, providing an accessible understanding of matter that appealed to the intellect of a diverse audience. His theory catalyzed a profound fascination with atomic structure and chemical interactions, inviting us to ponder our existence within a universe governed by atomic behavior. As we navigate the ever-evolving landscape of scientific discovery, Dalton’s insights serve as a reminder of the beauty and complexity embedded within the very fabric of reality.