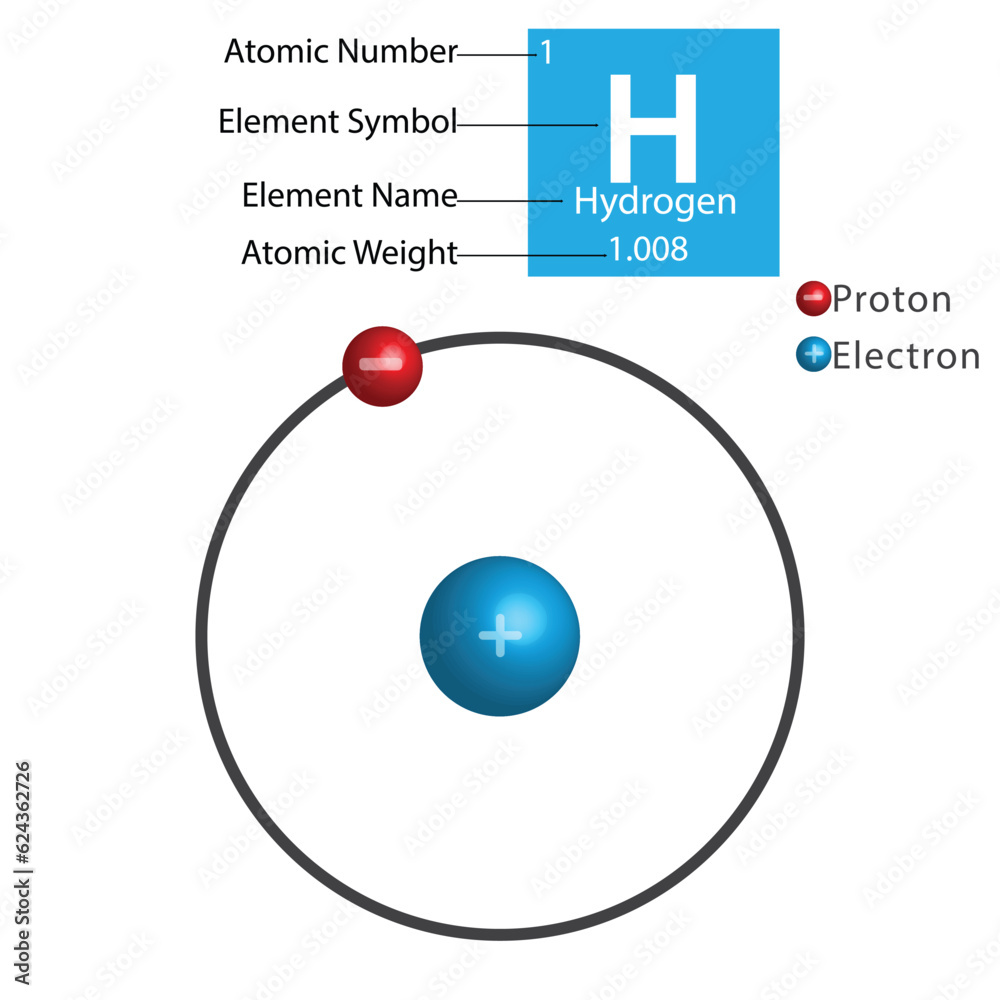
The hydrogen atom, the simplest and most abundant element in the universe, presents a fascinating glimpse into the realm of atomic structure. Comprised of just one proton and one electron, hydrogen serves as a fundamental building block for understanding atomic theory and the intricacies of matter. This article delves into the atomic structure of a hydrogen atom, exploring its constituents, behavior, and implications for broader scientific concepts.
At its core, the hydrogen atom is defined by its constituent parts: a solitary proton in the nucleus and an accompanying electron in a probabilistic cloud around it. The proton, with a positive charge of +1 elementary charge, occupies a minuscule volume at the center of the atom, composing the nucleus. In contrast, the electron, which carries a negative charge of -1 elementary charge, resides in a dynamic orbital that reflects the principles of quantum mechanics.
The electron does not possess a fixed position; rather, its location is described by a probability density function, illustrating the concept of electron orbitals. This notion diverges from classical representations of planetary orbits. Instead of a defined path, the electron’s behavior can be modeled through quantum wave functions, which provide an elegant yet complex understanding of its distribution. The vagueness of the electron’s position raises profound ramifications for the interpretation of atomic interactions and chemistry itself.
In examining the nucleus, one must consider the role of the strong nuclear force. This fundamental interaction binds protons and neutrons together within atomic nuclei, primarily evident in more complex elements. However, hydrogen, as the simplest atom, emphasizes the proton’s pivotal role without the presence of neutrons. The attractive forces between the positively charged proton and negatively charged electron generate the cohesive stability necessary for the atom’s integrity. This balance signifies a foundational principle in atomic theory: the intricate dance between attraction and repulsion.
Beyond the structural aspects, the electron’s energy levels and transitions provide essential insight into the behavior of hydrogen. Electrons occupy discrete energy levels or shells, dictating their energy states. When energy is imparted to an electron, whether through thermal stimuli, electromagnetic radiation, or collisions with other particles, it may be excited to a higher energy level. This form of excitation allows for intriguing phenomena such as emission and absorption spectra, which serve as fingerprints for elements in analytical chemistry.
The emission spectrum, resulting from electrons reverting to lower energy states, presents distinct lines unique to hydrogen. Each spectral line corresponds to a specific energy transition, providing compelling evidence for quantum mechanics and the quantization of energy levels. Conversely, the absorption spectrum reveals the wavelengths of light absorbed by electrons as they transition to higher energy states. Hydrogen’s emission and absorption spectra have been pivotal in astrophysical observations, aiding the identification of hydrogen in distant stars and galaxies.
Furthermore, the hydrogen atom serves as a cornerstone for discussions surrounding isotopes, providing a broader understanding of atomic variance. The existence of deuterium (hydrogen with one neutron) and tritium (hydrogen with two neutrons) introduces the notion of isotopic composition and stability. These isotopes exhibit similar chemical properties but profoundly different physical characteristics. This concept hints at the multidimensional nature of elemental behavior, contributing to a more nuanced understanding of matter.
Moreover, the simplicity of hydrogen makes it an ideal subject for theories addressing atomic interactions and bonding, exemplified in the formation of molecules. In molecular hydrogen (H₂), two hydrogen atoms covalently bond through the sharing of their electrons. This bond formation is a testament to the interplay of quantum mechanics and thermodynamics, elucidating how fundamental particles cooperate to yield complex systems. The exploration of hydrogen bonding extends beyond simple molecules and plays a significant role in the stability and properties of larger organic compounds, impacting fields ranging from biochemistry to materials science.
Hydrogen’s role in the cosmos cannot be overstated. Forming stars through nuclear fusion processes, hydrogen serves as the primary fuel for stellar lifecycles. The transformation of hydrogen into helium within the core of stars releases immense energy, underpinning the luminosity of celestial bodies. This phenomenon not only contributes to the stability of stars but is also crucial in generating the heavier elements through nucleosynthesis, thus shaping the universe itself.
In conclusion, the atomic structure of a hydrogen atom, though deceptively simple, invites a multitude of inquiries and perspectives. From its fundamental components to the complex principles governing its behavior, the hydrogen atom illustrates the intricate interplay of forces and energies that define our physical world. Its relevance extends beyond mere atomic theory, impacting various scientific disciplines, including chemistry, physics, and astronomy. Embracing the wonders of the hydrogen atom fosters a deeper appreciation for the universe’s underlying architecture, encouraging a quest for knowledge that transcends traditional boundaries.