Chemical bonding is a fundamental concept in chemistry, encapsulating the interactions that allow atoms to combine and form matter. Understanding the intricacies of chemical bond formation provides insights not only into the behavior of elements but also into the formation of compounds that define the material world.
When discussing chemical bonds, it is essential to differentiate between the primary types: ionic, covalent, and metallic bonds. Each type of bond has its unique characteristics and methods of formation, shaping the properties of the resultant compounds.
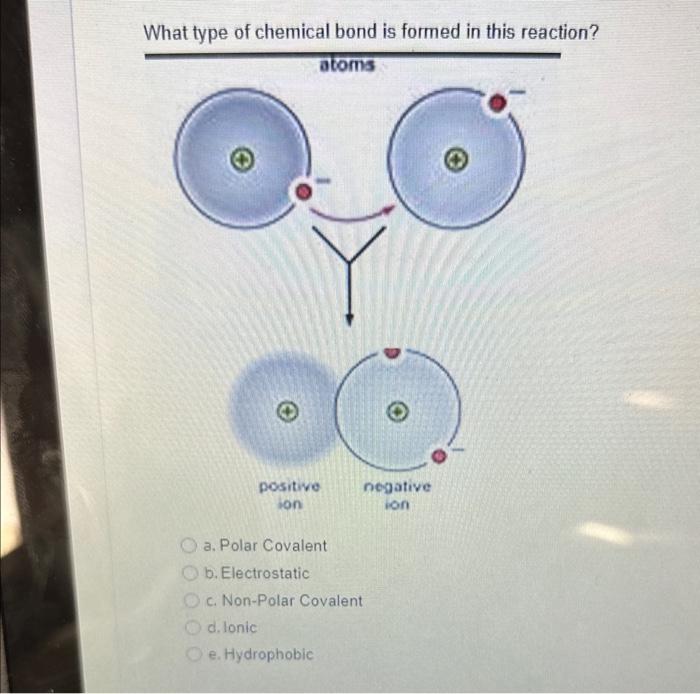
Ionic Bonds: Ionic bonding occurs when one atom transfers electrons to another atom, resulting in the formation of oppositely charged ions. This process typically involves a metal and a non-metal. The metal, having a low electronegativity, relinquishes one or more of its electrons to achieve a stable electron configuration, often resembling the nearest noble gas. Consequently, it becomes a positively charged cation. The non-metal, with a higher electronegativity, gains these electrons, becoming a negatively charged anion.
This electron transfer not only stabilizes the individual ions but also leads to a strong electrostatic attraction between them, which is the hallmark of ionic compounds. An archetypal example is sodium chloride (NaCl), where sodium donates an electron to chlorine. The resulting ionic lattice structure imparts distinctive properties to ionic compounds, such as high melting and boiling points, electrical conductivity in solution, and brittleness.
Covalent Bonds: Covalent bonding diverges from ionic bonding in that it involves the sharing of electron pairs between atoms. This type of bond predominantly occurs between nonmetals, allowing the participating atoms to achieve a full valence shell through mutual sharing, thereby stabilizing themselves.
The shared electrons create a bond that can be characterized as a simple single bond (one pair shared) or more complex arrangements such as double or triple bonds (two or three pairs shared, respectively). An illustrative example includes the formation of water (H2O), where each hydrogen atom shares an electron with oxygen, forming a bent molecular geometry critical to water’s unique properties, such as its high specific heat and solvent capabilities.
Moreover, covalent bonding can lead to the formation of polar and nonpolar molecules, dependent upon the electronegativity of the involved atoms. Polar covalent bonds arise when there is a significant difference in electronegativity, resulting in a dipole moment; this is prominently illustrated in molecules like HCl (hydrochloric acid).
Metallic Bonds: Metallic bonding, distinctive in its nature, occurs in metals due to the presence of a ‘sea of electrons.’ In this scenario, metal atoms donate their valence electrons to a shared pool, creating a lattice of positively charged metal cations that are held together by these delocalized electrons. This unique bonding paradigm affects metals’ physical properties, including their malleability, ductility, and thermal and electrical conductivity.
The structure of metallic bonds allows for the effective movement of electrons, which explains why metals can conduct electricity efficiently. Notably, the malleability of metals can be attributed to the ability of metal ions to slide past one another without breaking the metallic bond, maintaining electrical neutrality.
Beyond merely identifying and classifying these bonds, it is paramount to delve into the implications of bond formation on the properties of substances. The energy changes associated with bond formation are substantial; during the bond formation process, energy is released, typically indicating an exothermic reaction. Conversely, bond dissociation requires the input of energy, rendering it an endothermic process.
This energetic interplay is encapsulated in the concept of bond enthalpy, which quantitatively measures the strength of a chemical bond. Stronger bonds possess higher bond enthalpy values and are less likely to break under thermal or mechanical stress, while weaker bonds demonstrate lower values and can be more readily disrupted.
The consequences of bond formation extend beyond chemical reactions; they influence the physical state of materials. For instance, covalent bonding results in molecular compounds that may exist as gases, liquids, or solids at room temperature, governed by the dipole-dipole interactions, hydrogen bonding, or van der Waals forces between molecules.
In stark contrast, the rigid ionic lattices formed through ionic bonding typically culminate in crystalline solids with defined geometric patterns, evident in halite and quartz, impacting their physical properties, such as solubility and hardness.
Ultimately, the formation of chemical bonds not only elucidates the interactions between individual atoms but also lays the groundwork for understanding the vast array of substances that constitute our universe. From the biological macromolecules that underpin life to the innovative materials propelling technological advancements, the principle of bond formation is integral to both the microscopic and macroscopic worlds.
In conclusion, the phenomena underlying chemical bond formation are multifaceted and critical for explaining the behaviors of compounds and materials. Through the lens of ionic, covalent, and metallic bonds, it becomes evident how these fundamental principles govern the properties and interactions of matter, thereby influencing all branches of science and industry. The study of chemical bonding remains a cornerstone of chemistry, providing a foundation upon which further investigations into molecular interactions and innovative applications can flourish.