Copper is a notable element in the periodic table, classified as a metal with a distinct atomic structure and versatile properties. To address the query, “Is copper an atom or a molecule?” we must first delineate the fundamental distinctions between atoms and molecules, and then analyze the intrinsic characteristics of copper in conjunction with these definitions.
At its core, an atom is the smallest unit of matter that retains the properties of an element. Each atom consists of a nucleus, which houses protons and neutrons, surrounded by a cloud of electrons. The arrangement of these subatomic particles determines the chemical behavior and physical properties of the element. Conversely, a molecule is a combination of two or more atoms chemically bonded together. These bonds may occur in various forms—covalent, ionic, or metallic—and result in the formation of distinct compounds or larger structures.
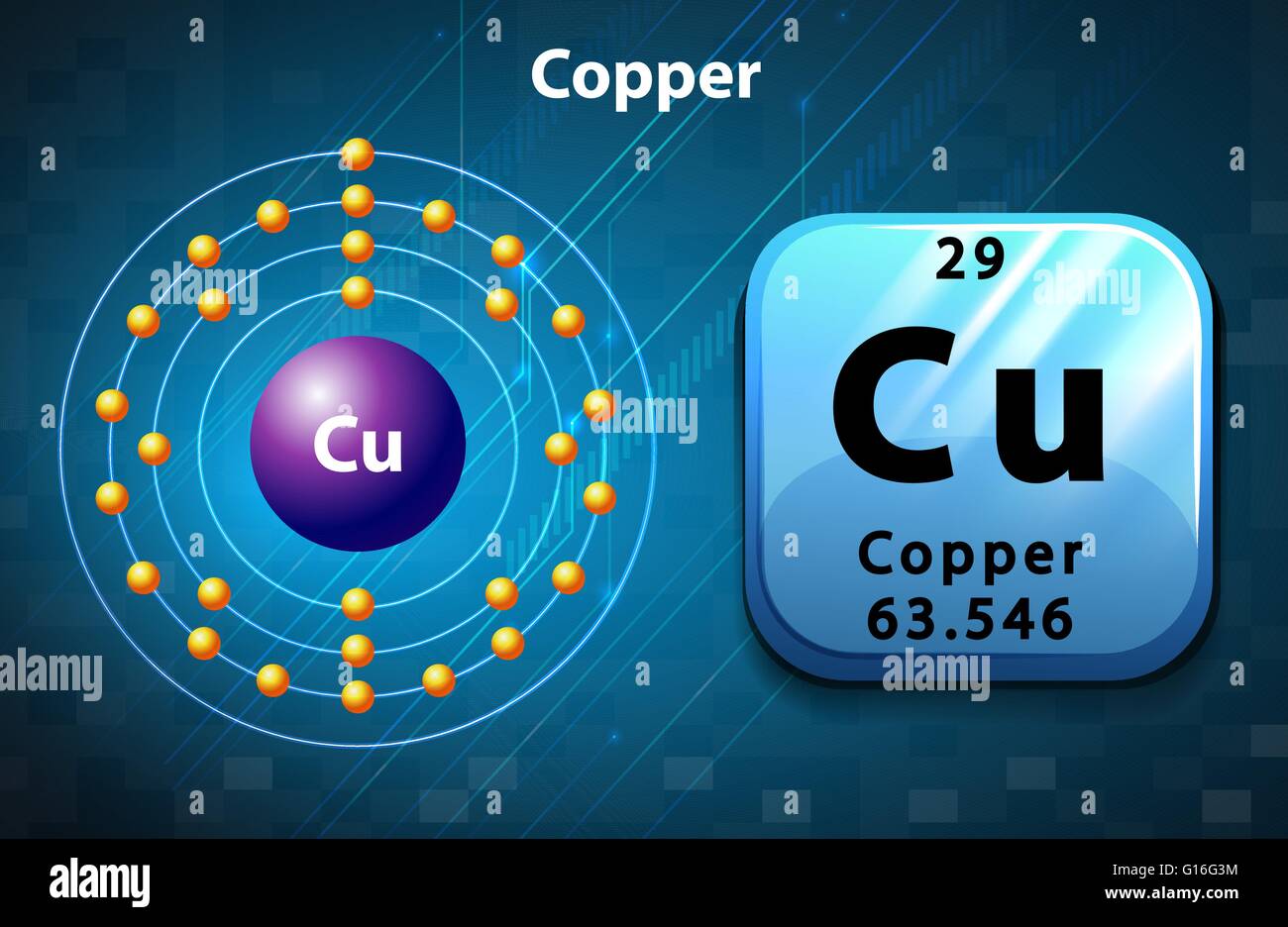
Copper (Cu), with an atomic number of 29, is an element characterized by its unique atomic structure. As an individual atom, copper possesses a specific number of protons (29), neutrons, and electrons that align in a manner that defines its identity. The electron configuration of copper is [Ar] 3d10 4s1, indicating a filled d-subshell and a single electron in the outermost s-subshell. This configuration is pivotal in understanding copper’s extensive applications, as it allows the metal to exhibit excellent electrical and thermal conductivity, malleability, and ductility.
Considering the aforementioned definitions, it becomes clear that copper, in its intrinsic form, is classified as an atom. However, the natural world often witnesses copper atoms amalgamating into larger structures, yielding a spectrum of copper-based compounds and minerals. For instance, copper(II) sulfate (CuSO4) is a notable compound wherein copper atoms bond with sulfate ions to form a molecule. This highlights the duality of copper—while it fundamentally exists as an atom, it can also participate in the formation of molecules.
To further illuminate the subject, it’s essential to explore copper’s behavior at the atomic and molecular levels within various contexts, such as its role in biological systems, its applications in technology, and its environmental impact.
In biological systems, copper serves as a vital micronutrient. It is integral to various enzymatic reactions, playing a crucial role in processes such as iron metabolism, collagen synthesis, and neurotransmitter function. The presence of copper in proteins like ceruloplasmin and cytochrome c highlights its molecular significance, bridging the gap between its atomic identity and its contributions to complex biological functions. Such interactions exemplify how a singular atom can influence intricate biochemical pathways and overall health.
Transitioning to technological applications, copper’s atomic properties lend it unparalleled utility in electrical engineering and electronics. Copper wires are ubiquitous in the electrical industry due to their low resistivity and high thermal conductivity. The formation of alloys, such as bronze and brass, involves the combination of copper with other metals, exhibiting the molecule’s essential role in enhancing specific material properties. Such amalgamations amplify the significance of copper atoms, transforming them into molecules that define our technological landscape.
On a broader scale, the environmental implications of copper must also be scrutinized. While copper is a naturally occurring element, its mining and processing can lead to detrimental ecological effects. Excess copper in the environment can be toxic to aquatic life, disrupting ecosystems and causing bioaccumulation. This environmental perspective further emphasizes the dual nature of copper as both an individual atom and a component of larger molecular structures that can have profound effects on ecosystems.
Moreover, copper’s historical relevance cannot be overstated. It has been utilized by humans for millennia, evolving from primitive tools to modern-day electrical systems. The age of copper, often heralded as a pivotal point in human technological advancement, showcases not only the historical significance of the element itself but also the collective effort of countless atoms merging to form functional tools and innovations.
In conclusion, the examination of copper through the lens of atomic and molecular science reveals a rich tapestry of interaction and versatility. Copper, as an atom, serves as a fundamental building block, while its ability to form molecules enhances its relevance in biological systems, technology, and environmental science. The question of whether copper is an atom or a molecule provokes curiosity about the interplay between these two states of matter, encouraging a deeper appreciation for the atom’s role in the grand scheme of chemistry and life itself. It invites scholarly contemplation of how single units aggregate to produce complex phenomena—an idea that resonates well beyond the realm of copper.