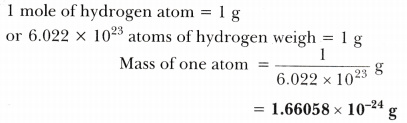Understanding the mass of a single atom of hydrogen is a fundamental inquiry in the realms of chemistry and physics. It bridges the macroscopic observations of matter with the microscopic entities that constitute it. Hydrogen, the most abundant element in the universe, serves as an essential building block for various molecules and plays a pivotal role in numerous chemical reactions. This exploration provides critical insights into atomic mass, its calculation, and implications in various scientific domains.
The Concept of Atomic Mass
Atomic mass, or atomic weight, refers to the weighted average mass of an element’s isotopes, measured in atomic mass units (amu). A single hydrogen atom is primarily composed of one proton and, in its most prevalent isotope, a solitary electron. This simplicity allows for easier calculations and provides a basis for comparison with more complex atoms. The atomic mass of hydrogen is approximately 1.008 amu, indicating that it is the lightest of all elements.
The Composition of Hydrogen
Hydrogen exists predominantly as three isotopes: Protium (¹H), Deuterium (²H), and Tritium (³H). Protium is the most common, constituting roughly 99.98% of all hydrogen found in nature. It consists of one proton and no neutrons. Deuterium, with one neutron, accounts for about 0.02%, while Tritium, a radioactive isotope with two neutrons, is relatively scarce. The presence of neutrons contributes marginally to the mass, thus influencing the weighted average atomic mass of hydrogen.
Calculating the Mass of One Hydrogen Atom
To determine the mass of a single hydrogen atom, one must first comprehend the concept of the atomic mass unit. One atomic mass unit is defined as one twelfth of the mass of a carbon-12 atom, approximately 1.66 x 10-27 kg. Therefore, the mass of a hydrogen atom can be detailed as follows:
- Mass of Protium (¹H) = 1.008 amu = 1.67 x 10-27 kg
- Mass of Deuterium (²H) = 2.014 amu = 3.34 x 10-27 kg
- Mass of Tritium (³H) = 3.016 amu = 5.03 x 10-27 kg
The simplicity in these measurements allows for the realization that even the slightest variations in isotopic composition can significantly affect the overall atomic mass of hydrogen in chemical reactions and molecular formations.
Hydrogen in the Context of Chemistry
In chemical bonding, the mass of hydrogen atoms plays an influential role. For instance, in water (H₂O), two hydrogen atoms combine with one oxygen atom. The respective masses guide stoichiometric calculations, essential for predicting the outcomes of chemical reactions. Additionally, hydrogen’s role as a reducing agent in many synthesis processes highlights its atomic weight’s importance, affecting reaction rates and products.
The Role of Hydrogen in Biological Systems
In biological systems, hydrogen atoms are integral components of biomolecules. Hydrocarbons, amino acids, proteins, and nucleic acids all feature hydrogen as a fundamental constituent. The mass of hydrogen impacts molecular weight calculations that are crucial for biochemistry—especially in metabolic pathways where enzymes facilitate reactions involving hydrogen transfer. The lightness of hydrogen enables swift mobility in biological processes, influencing cellular respiration and photosynthesis.
Interstellar Hydrogen: A Cosmic Perspective
The mass of hydrogen atoms also holds astronomical significance. In the cosmos, hydrogen constitutes a substantial fraction of baryonic matter, forming stars and galaxies. The nuclear fusion processes within stars convert hydrogen into helium, enlightening the origins of element formation and contributing to the chemical evolution of the universe. Understanding hydrogen’s mass and behavior is vital for astrophysics and the study of cosmological phenomena.
Precision Measurement and Technological Advances
Advancements in technology have augmented the precision with which scientists can measure atomic mass. Techniques such as mass spectrometry enable the determination of atomic weights with remarkable accuracy, paving the way for innovative research in fields ranging from nanotechnology to materials science. As methodologies evolve, the implications of these measurements will likely expand, fostering further discoveries in both theoretical and applied sciences.
Conclusion: The Significance of Hydrogen’s Mass
In conclusion, the mass of one atom of hydrogen may seem minuscule—approximately 1.67 x 10-27 kg—but its implications are profound and far-reaching. From its fundamental role in chemical reactions to its pivotal presence in biological systems and the cosmos, hydrogen’s atomic mass elucidates essential truths about matter itself. Key insights into its behavior not only influence theoretical frameworks but also practical applications across various scientific disciplines.