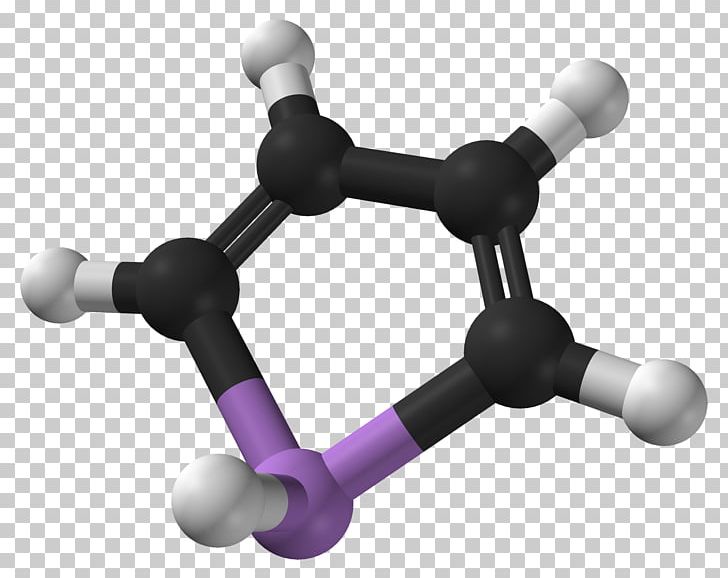
The field of chemistry is replete with fascinating molecular constructs, and one such intriguing compound is arsole. Arsole, a member of the class of organoarsenic compounds, features a five-membered aromatic ring that incorporates arsenic in place of one of the carbon atoms typically present in similar cyclic structures such as pyrrole. Although the material is less commonly encountered than its carbon counterparts, the study of its molecular orbitals opens a window into the complexity and beauty of chemistry. This exploration will delve into the molecular orbital configuration of arsole, providing insight into the underlying physical principles and chemical implications.
To comprehend the molecular orbitals of arsole, one must first revisit the concept of hybridization and the formation of molecular orbitals. The electronic configuration of arsenic, which has an atomic number of 33, is [Ar] 3d10 4s2 4p3. In arsole, the arsenic atom substitutes for a carbon in a traditional aromatic system, leading to a distinct hybridization state. Specifically, the central atom in arsole typically exhibits sp2 hybridization. This hybridization involves the mixing of one s orbital and two p orbitals to create three equivalent sp2 hybrid orbitals, which are oriented in a planar geometry. Each of the remaining p orbitals can participate in π bonding, filling out the aromatic system.
The structural framework of arsole is illustrated by the shared electron density within the π molecular orbitals that comprises the five-membered ring. In more familiar terms, this structure influences not only the magnetic resonance properties but also indicates the stability of the compound due to its aromatic characteristics. The conjugation found in arsole leads to the delocalization of the π electrons, stabilizing the system and contributing to its chemical behavior.
Furthermore, the molecular orbital configuration in arsole can be dissected into bonding, anti-bonding, and non-bonding orbitals consequent upon the principles of quantum mechanics and the Pauli exclusion principle. The bonding orbitals are characterized by lower energy levels, enhanced stability, while the anti-bonding orbitals are positioned at higher energy levels and are typically occupied only in excited states or can hold no electrons under ground state conditions.
Visually, the molecular geometry of arsole, due to sp2 hybridization, resembles a flattened pentagon, with bond angles close to 120 degrees, indicative of its trigonal planar character in the regions surrounding the sp2 hybridized arsenic atom. This configuration facilitates the overlap of the p orbitals with adjacent p orbitals on carbon atoms. These overlapping p orbitals create a continuous π system, resulting in the delocalization of electrons around the ring.
Another pivotal aspect of the arsole orbital structure involves the consideration of symmetry. When analyzing the molecular orbitals, the application of group theory can provide profound insights into the practical symmetry of the compound. Arsole’s symmetry properties allow the classification of its molecular orbitals into specific symmetry species, greatly affecting the electronic transitions and the optical properties of the molecule. Such transitions are crucial for understanding the molecule’s reactivity and interaction with various electromagnetic radiation, leading to further insights into spectroscopic behavior.
The presence of the arsenic atom in the molecular structure also introduces unique electronic characteristics that set arsole apart from its organic counterparts. For instance, the electronegativity of arsenic affects the energy levels of the molecular orbitals and alters the electronic activity of the entire molecule. The inherent ability of arsole to engage in coordination chemistry due to the presence of the p orbitals within arsenic comes with added potential for its application in catalysis and materials science.
The study of arsole and its molecular orbitals virtually serves as an epitome of the interplay between structure and function within chemical compounds. The unique properties of arsole make it a subject of interest not only for its inherent chemical behavior but also for its broader implications in understanding organoarsenic chemistry. The precise arrangement of its molecular orbitals imparts specific reactivity patterns that can have significant impacts in organic synthesis and drug design.
In conclusion, the molecular orbitals of arsole present an exquisite example of the complexity of hybridized structures in chemistry. By interlacing principles of quantum mechanics with fundamental organic chemistry concepts, the distinctive electronic properties and myriad implications of arsole become apparent. As such, further exploration into the molecular orbitals of arsole can yield fruitful possibilities, inviting chemists to unveil a greater understanding of this intriguing class of compounds. The narrative of arsole transcends mere structure — it beckons for continuous inquiry into its myriad applications and underlying molecular principles, challenging conventional perceptions within the realm of chemistry.