In the realm of chemistry and physics, the terms “elements” and “atoms” often arise in discussions surrounding the fundamental building blocks of matter. However, while these terms are sometimes utilized interchangeably in colloquial contexts, there exists a fundamental distinction between the two. Understanding this differentiation is pivotal for grasping the complexities of atomic theory and the composition of matter.
To elucidate this point, we first explore the definition of an element. An element is a pure substance that cannot be decomposed into simpler substances by chemical means. Each element is characterized by a unique atomic number, which corresponds to the number of protons found in the nucleus of its atoms. The periodic table serves as the quintessential repository of known elements, categorizing approximately 118 distinct entities based on their atomic structure and properties. Elements such as hydrogen (H), carbon (C), and gold (Au) are familiar examples of substances that exist in nature in elemental form, each represented by one or two-letter symbols.
In contrast, atoms represent the smallest unit of an element that retains its chemical properties. An atom consists primarily of a nucleus, composed of protons and neutrons, surrounded by a cloud of electrons. This atomic structure defines the reactivity and chemical behavior of the element. For instance, the element carbon exists in nature not only as individual carbon atoms but also in various forms such as graphite and diamond—two allotropes with distinct physical properties. Herein lies the crux of the distinction: while elements pertain to the substances themselves, atoms denote the minute constituents that comprise these substances.
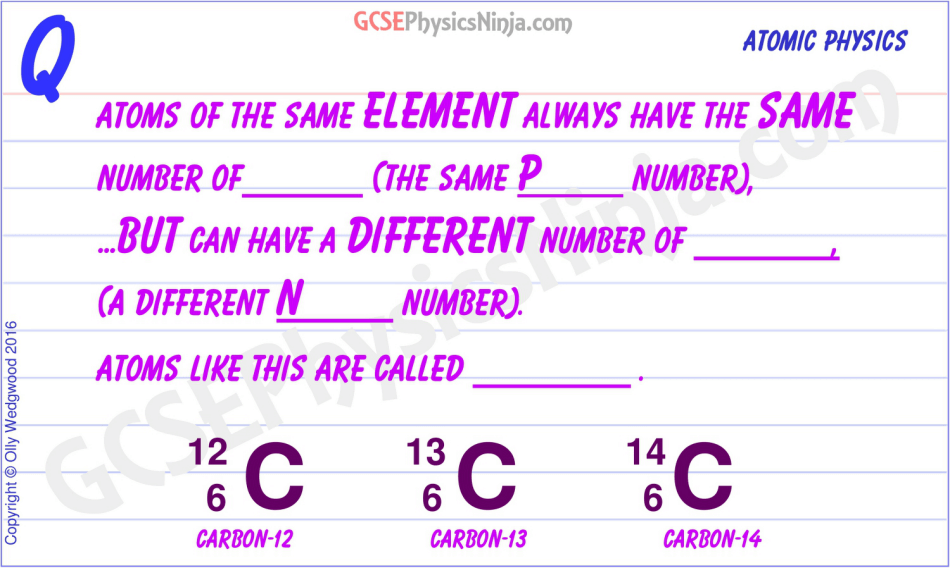
Delving deeper into the realm of atomic structure, we discover isotopes—variations of a particular element that have the same number of protons but differing numbers of neutrons. This variability introduces nuances in the behavior and stability of elements. For example, carbon has isotopes such as carbon-12 and carbon-14, which possess six and eight neutrons, respectively. The presence of these isotopes further accents the fact that while the element remains unchanged, the atomic variation can yield different physical and nuclear properties, affecting applications in fields such as radiocarbon dating and medical imaging.
It is crucial to recognize that elements may exist in multiple ionic or molecular forms as well. For instance, chlorine exists both as Cl₂ gas (diatomic molecule) and as chloride ion (Cl⁻) in ionic compounds, highlighting the adaptability of atoms when combined with other elements or in different states. This adaptability underscores the diversity of chemical compounds and reactions, where elements engage in bonding through covalent, ionic, or metallic interactions to form complex structures, further enriching the understanding of chemical dynamics.
Another vital concept is the distinction between intrinsic and extrinsic properties associated with elements and atoms. Intrinsic properties—such as atomic mass, melting point, and density—are inherent to the element, whereas extrinsic properties may arise from the interactions of atoms in a specific context. For instance, the element sodium (Na) is a soft, reactive metal in its elemental form. However, when it forms sodium chloride (NaCl), the properties shift to those of a stable ionic compound, showcasing how the interplay of atoms can lead to dramatic transformations in behavior.
Moreover, modern advancements in the field of quantum mechanics have redefined our understanding of atomic behavior, introducing the concept of electron orbitals and their probabilistic nature. This quantum perspective emphasizes that atoms are not static entities but rather engage in dynamic interactions with their environment, influencing chemical behavior and bonding arrangements. Each atom’s electron configuration plays a critical role in determining how an element will react with others, offering a more profound comprehension of matter at the molecular level.
Furthermore, the implications of distinguishing between elements and atoms extend into practical realms such as material science, molecular biology, and nanotechnology. Knowledge about the atomic composition and the behavior of different elements guides researchers in designing novel materials with specific properties, as well as understanding biological mechanisms at the cellular level. By controlling atomic interactions, scientists can engineer substances with unprecedented characteristics, highlighting the critical nature of this distinction in real-world applications.
In conclusion, while elements and atoms may share an intrinsic connection as fundamental components of matter, their definitions and implications diverge significantly. Elements represent the pure substances that cannot be broken down, while atoms are the individual units that embody the properties of those elements. Recognizing the nuances within this relationship not only clarifies misunderstandings but also enriches our comprehension of the material world. As science continuously unravels the complexities of atomic interactions, the distinction between elements and atoms remains a cornerstone of both theoretical exploration and practical application in various scientific domains.