Atoms: the fundamental building blocks of matter. They are the tiny, invisible units that compose everything around us, from the air we breathe to the stars that illuminate our night sky. As we delve into the composition and stability of these minuscule entities, the question arises: Are atoms held together by magnetism? This inquiry presents an intriguing challenge for both novice learners and seasoned physicists alike, as it compels us to explore the intricate forces at play within atomic structures.
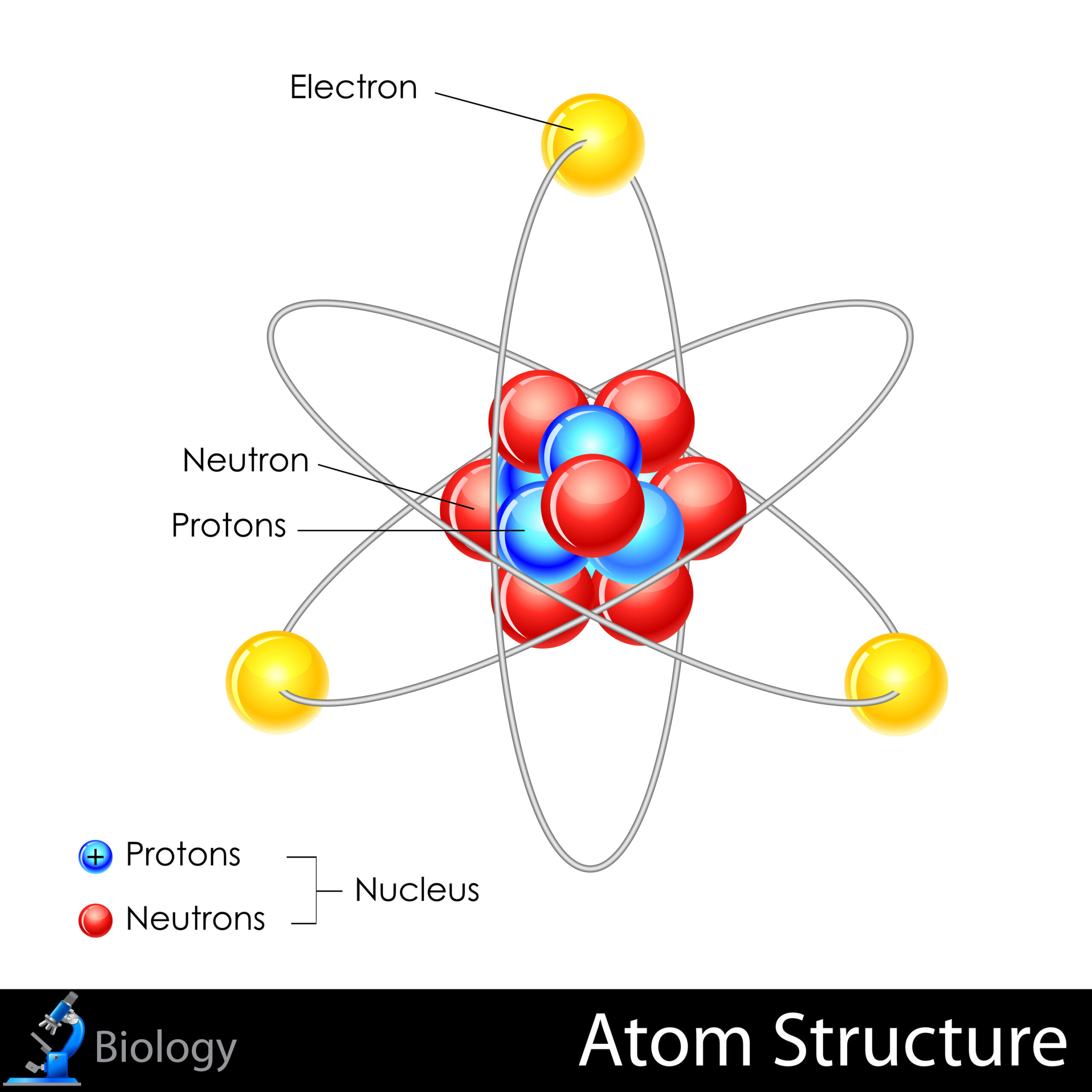
To address this question, we must first dissect the atomic architecture. Atoms are primarily composed of a nucleus, which contains positively charged protons and neutrally charged neutrons, enveloped by a cloud of negatively charged electrons. The electrons orbit the nucleus in defined energy levels, exhibiting behaviors that are often analogous to celestial bodies in orbit — albeit governed by quantum mechanics instead of classical physics.
At first glance, one might be tempted to liken the interactions that maintain atomic integrity to magnetism. After all, magnetic forces are prominent in numerous physical phenomena. However, this comparison warrants a closer examination, as the forces that bind atoms are fundamentally different from magnetic attraction.
In atomic structures, the predominant force is actually the electromagnetic force, a cornerstone of the Standard Model of particle physics. The electromagnetic force functions to attract oppositely charged particles — the electrons and protons — thereby conferring stability to the atom. The interplay of electromagnetic forces stands in stark contrast with magnetism, which emerges from the movement of charged particles — specifically, the alignment of magnetic dipoles within materials.
To further complicate the matter, one must consider the nature of the strong nuclear force, a critical player in maintaining the integrity of the atomic nucleus. This force is substantially stronger than electromagnetic forces, but it operates over a much shorter range. It effectively binds protons and neutrons together within the nucleus, overcoming the electromagnetic repulsion between like-charged protons. This intricate dynamic is vital for the stability of atomic structures; without the strong force, atomic nuclei would succumb to disintegration.
Thus far, we have delineated the distinction between attractive electromagnetic forces and the formidable strong nuclear force. Yet, this raises a further question: Where does magnetism fit into the grand scheme of atomic interactions? While it does not play a direct role in holding atoms together, magnetism manifests in subtler ways. For instance, it influences the behavior of materials at a macroscopic level through phenomena such as ferromagnetism, diamagnetism, and paramagnetism, which originate from the arrangement of electrons and their spins within atoms.
This brings us to a pivotal point of understanding: while magnetism pertains to interactions between larger aggregates of atoms or between atomic dipoles, it is not the fundamental force responsible for the cohesion of atoms themselves. The magnetic properties of a substance originate when many atoms exhibit cooperative behaviors, such as aligned spins, that aggregate to produce observable magnetic fields.
Let us turn our attention to the atomic scale and understand electron configurations. Electrons reside in distinct orbitals, associated with specific energies, and exhibit behaviors informed by quantum mechanics. The Pauli Exclusion Principle prohibits two electrons from occupying the same quantum state, leading to intricate arrangements that affect the magnetic properties of materials. In certain cases, unpaired electrons in an atom may denote a magnetic character, thus allowing magnetism to manifest only when collating a multitude of atoms.
Nevertheless, this does not imply that magnetism plays a role in holding individual atoms together; it merely provides a fascinating glimpse into the broader implications of atomic interactions. The confinement of electrons within their respective orbitals, driven primarily by electromagnetic forces, along with the bind of nucleons through the strong nuclear force, stands as the definitive answer to whether atoms are held together by magnetism.
In conclusion, while magnetism is a captivating facet of physical science, it does not serve as a cohesive force at the atomic level. Electromagnetic and strong nuclear forces are the stalwarts of atomic stability, diligently working to maintain the structure of matter as we know it. This exploration highlights the complexity of atomic interactions and underscores the importance of distinguishing between different forces at play in the universe.
Ultimately, the inquiry into the nature of atomic cohesion leads us to appreciate the remarkable intricacies of the atomic world. While magnetism remains an exhilarating phenomenon in its own right, the fundamental forces that govern atomic structure invite us to ponder further. How do these concepts interrelate? What implications do they hold for the expansive universe of matter? In seeking answers, we embark on a perpetual journey through the realms of physics, where each discovery leads to more profound questions and a deeper understanding of the cosmos.