At the atomic scale, the universe presents a fascinating tableau characterized by forces and interactions that shape the very fabric of matter. Among these, atomic attraction stands as a principal force, dictating the behavior of atoms and molecules in their quest to achieve stability. What would happen if we could perceive the forces acting on a single atom? Would it be akin to experiencing gravitational pull on a cosmic scale, or might it derive from a more nuanced perspective informed by quantum mechanics?
To delve into the essence of atomic attraction, it is essential to begin with an understanding of the primary forces that govern atomic interactions. Atoms are composed of protons, neutrons, and electrons, constituting a complex interplay of electromagnetic and nuclear forces. The electromagnetic force emerges from the interactions between charged entities; in the case of atoms, it primarily involves the attraction between positively charged protons in the nucleus and negatively charged electrons orbiting around it. This fundamental electromagnetic interaction, known as Coulomb’s Law, forms the bedrock of atomic stability.
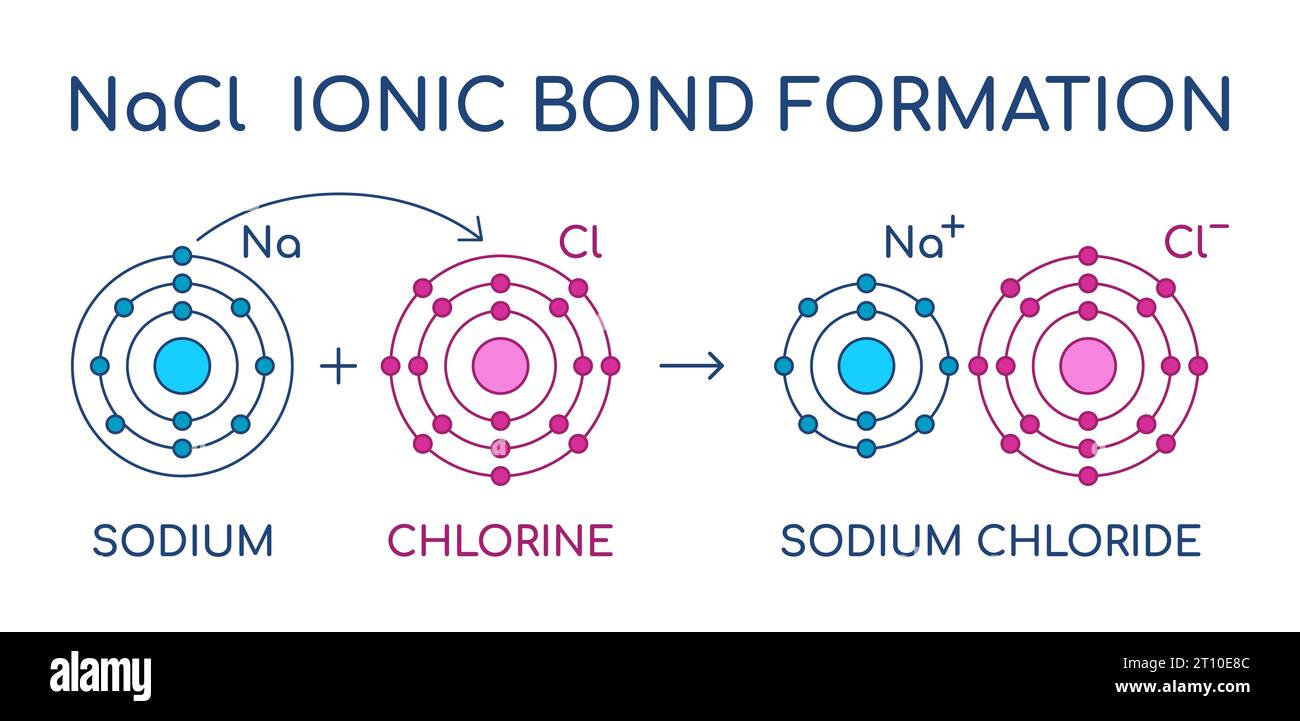
To illustrate, consider the case of sodium chloride (NaCl), commonly known as table salt. The formation of NaCl is emblematic of ionic bonding—a result of the transfer of electrons from one atom to another. Sodium (Na), a metal, donates an electron to chlorine (Cl), a non-metal. This electron transfer results in the formation of oppositely charged ions: Na+ and Cl–. The electrostatic attraction between these ions is a prime example of atomic attraction in action. Yet, this attraction is not merely a spatial phenomenon; it embodies a complex interaction that resonates with the principles of electron configuration and energy minimization.
It is essential to contextualize atomic attraction within the framework of quantum mechanics. At this scale, phenomena often defy classical intuition. Electrons do not occupy defined orbits around the nucleus; instead, they exist in probabilistic distributions known as electron clouds. This nuanced behavior complicates how we perceive atomic attraction. For instance, when two atoms approach each other, their electron clouds overlap, leading to the phenomenon known as shielding. When electrons from nearby atoms repel one another, this results in a balance of forces that ultimately determines whether the atoms will bond, share, or remain apart.
One might ask, how does atomic attraction contribute to the myriad of physical properties observed in materials? The answer lies in the distinction between different types of bonding: ionic, covalent, and metallic. Ionic bonds, as exemplified in NaCl, result in the formation of crystalline structures exhibiting high melting and boiling points due to the strength of the electrostatic forces between ions. On the other hand, covalent bonds arise when atoms share electrons, as seen in molecular compounds such as water (H2O). These shared electrons create localized bonds that can impart varying degrees of volatility and reactivity to substances. Furthermore, metallic bonds, characterized by a “sea of electrons,” allow metals to conduct electricity and exhibit malleability and ductility.
A playful inquiry into atomic attraction could invoke a thought experiment: Imagine a scenario in which a person could “feel” the molecular attractions and repulsions at play within a substance. How would it alter our understanding of material properties? Would it revolutionize fields such as materials science, where atomic-level manipulations could be guided by intuitive experiences rather than complex models? This proposition embodies both a playful curiosity and a profound challenge: how to create a tangible interface between human experience and the intricate world of quantum interactions.
Despite the inherent complexities, atomic attraction serves as a pivotal force in explaining thermal properties and phase transitions. As temperature increases, the kinetic energy of atoms rises, leading to greater atomic vibrations and movements. This increased motion can overcome the attractive forces between atoms, resulting in phase transitions from solid to liquid and liquid to gas. For instance, when ice melts to water, the orderly arrangement of water molecules is disrupted, revealing the balance of attractive forces that dictate states of matter.
At the molecular level, atomic attraction is further enriched by the concept of intermolecular forces, which include hydrogen bonding, dipole-dipole interactions, and London dispersion forces. Each of these interactions plays a pivotal role in determining the physical characteristics of substances. Hydrogen bonding, for example, is responsible for the unique properties of water, such as its high specific heat capacity and surface tension, which are crucial for sustaining life on Earth.
In summary, atomic attraction is more than a fundamental force; it is the thread that weaves together the intricate tapestry of matter. By comprehensively understanding the nuances of atomic interactions and their macroscopic manifestations, scientists can better grasp the complexities of the natural world. To fully appreciate the subtleties of forces at play, the challenge lies in reconciling our macroscopic experiences with the intricate quantum behavorial nuances experienced at the atomic level. This endeavor underscores the beauty and complexity of the universe, prompting deeper inquiries into the nature of matter and the forces that bind it together.