At the heart of modern science lies a profound inquiry that captivates not only physicists but also curious minds from various fields: Are atoms made up of particles or waves? This question delves into the fundamental nature of matter and energy, provoking a fascination that transcends beyond the mere constituents of the universe. It invites us to explore the very essence of reality and compels us to reconsider our perceptions of the microscopic world.
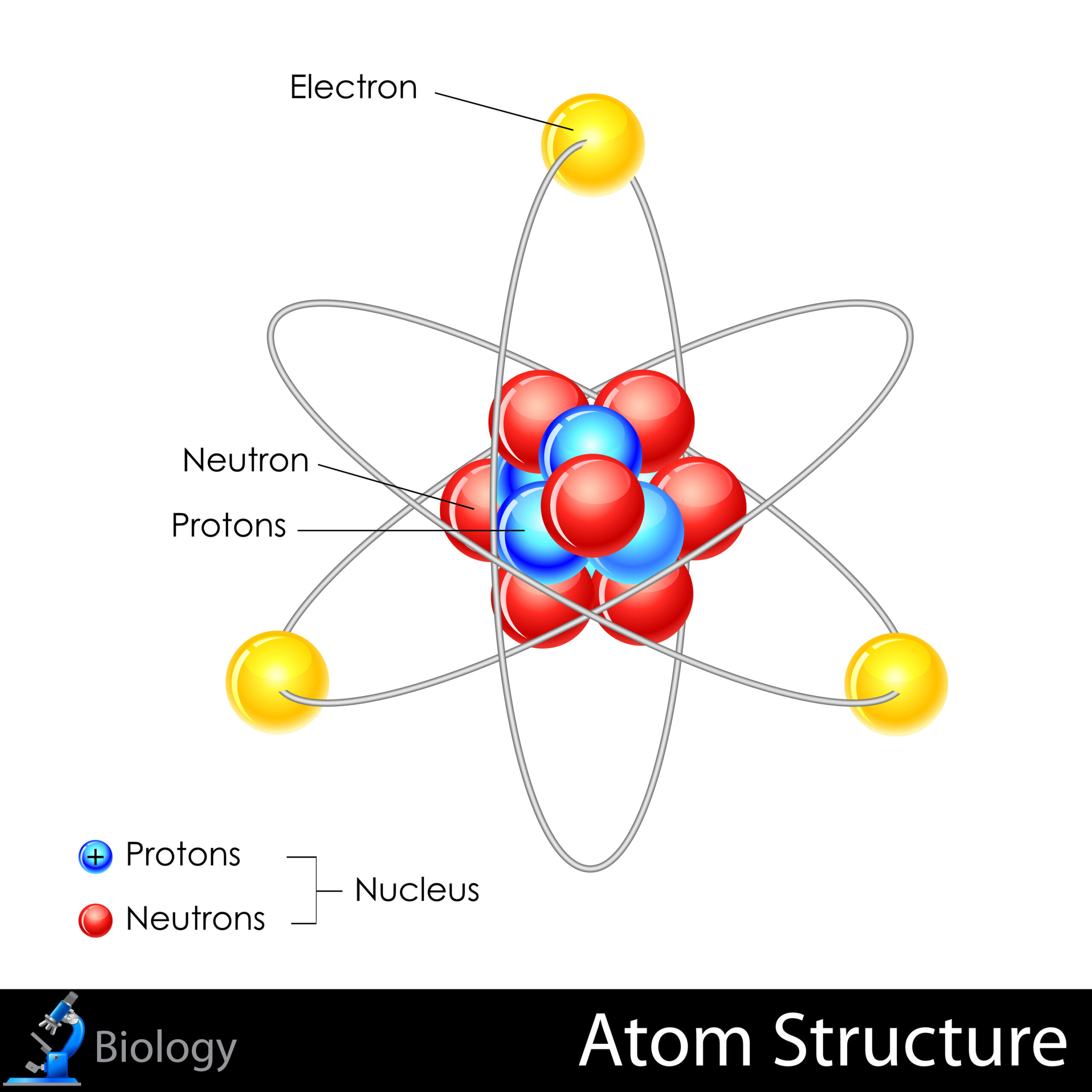
Traditionally, atoms have been understood as the building blocks of matter. The classical view posits that an atom consists of a nucleus, composed of protons and neutrons, surrounded by a cloud of electrons. This model paints a picture of a miniature solar system, where particles orbit their nucleus in defined paths. However, delving deeper into atomic structure unveils a more complex and intriguing narrative—one that integrates both particle and wave theories, giving rise to what is known as wave-particle duality.
Wave-particle duality is a cornerstone of quantum mechanics, challenging the dichotomy between particles and waves. According to this principle, entities such as electrons exhibit both particle-like and wave-like behaviors, necessitating a reevaluation of their fundamental nature. The landmark double-slit experiment poignantly illustrates this duality. When electrons are fired through two closely spaced slits, they create an interference pattern on a detecting screen, indicative of a wave-like behavior. However, if one attempts to measure which slit an electron traverses, the interference disappears, and electrons behave as discrete particles. This phenomenon underscores the elusive character of atomic constituents—one that is as paradoxical as it is captivating.
Furthermore, the wave function—a mathematical construct that embodies the probabilities of an electron’s position and momentum—intricately connects the particle and wave descriptions. Formulated by Erwin Schrödinger, the wave function encapsulates the inherent uncertainty that pervades quantum systems. Rather than offering a single, deterministic outcome, it provides a probabilistic interpretation, illustrating that the attributes of atomic particles exist in a state of superposition until measured. Such concepts challenge our classical intuitions and inspire awe at the randomness and complexity of the universe.
Delving into the microcosm of atomic structure, we encounter fascinating entities known as quarks, which are the fundamental constituents of protons and neutrons. Quarks themselves do not exist independently; instead, they are perpetually bound in clusters, interacting through the strong nuclear force. The intricate interplay among quarks results in the formation of composite particles, further deepening the complexity of atomic architecture. This raises interesting questions, such as whether quarks themselves exhibit wave-particle duality or if this behavior is a broader characteristic of all fundamental particles.
The implications of particles exhibiting wave-like behavior extend beyond theoretical discussions. In the realm of chemistry and materials science, understanding the wave properties of electrons is pivotal to elucidating phenomena such as conductivity, magnetism, and chemical bonding. Electrons do not merely orbit the nucleus; their wave-like functions dictate the arrangement and interactions that lead to the formation of complex molecules and materials. This interplay of wave and particle characteristics provides a framework to comprehend how the macroscopic world emerges from the atomic scale.
Moreover, the wave-particle nature of atoms unlocks intriguing avenues for technological advancement. Quantum mechanics paves the way for innovations such as quantum computing and quantum cryptography—fields that harness the principles of superposition and entanglement. These technologies, predicated on the dual nature of particles and waves, have the potential to revolutionize information processing and secure communication in ways previously unimaginable. The enthralling prospects of harnessing atomic behavior magnify the significance of comprehending whether atoms are fundamentally particles or waves.
As we navigate the complexities of atomic structure, it becomes evident that the question is not merely academic; it compels us to confront our understanding of reality itself. The interplay between particles and waves evokes a sense of wonder and invites deeper philosophical reflections. Can we distinguish between the observer and the observed? Does the act of measurement alter the very phenomena we seek to comprehend? The inquiries extend into the realm of ontology, where the boundaries of science blur with the intricacies of consciousness and perception.
In examining the nature of atoms, society grapples with broader implications. This investigation prompts us to reconsider our place in the universe. Are we not, in our essence, composed of the same atomic structures, oscillating in a symphony of waves and particles? The contemplation of our shared atomic heritage draws connections between quantum mechanics and the fundamental questions regarding life’s origins and the connectivity of all matter. Thus, the quest to answer whether atoms are made of particles or waves transcends the laboratory, echoing literally in the fabric of existence.
Ultimately, the query encapsulates both a scientific pursuit and a voyage of discovery. As physicists continue to unravel the enigma of atomic composition, one thing remains abundantly clear: the characteristics of atoms defy binary descriptions. Embracing their dual nature propels us forward into uncharted scientific territories, fostering a profound appreciation of the universe’s inherent complexities. The intrigue surrounding the duality of atoms serves as a testament to the endless curiosity that propels scientific inquiry and enriches the human experience.