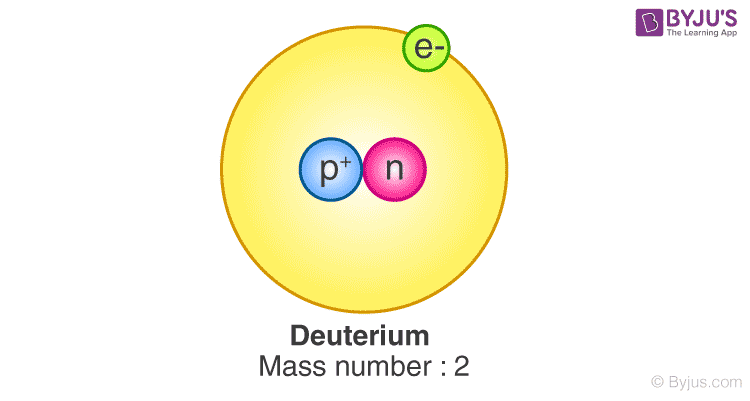
Deuterium, often denoted as D or ²H, is one of the two stable isotopes of hydrogen, the lightest element in the periodic table. This fascinating isotope possesses one proton and one neutron in its nucleus, making it approximately twice as heavy as its more prevalent counterpart, protium (¹H), which contains only a proton. The unique properties of deuterium have captured the attention of scientists, particularly in the fields of nuclear physics and chemistry, due to its significant role in various nuclear reactions.
To understand why deuterium is used in nuclear reactions, it is essential to explore its foundational characteristics. The presence of the neutron grants deuterium distinct nuclear properties, which influence its behavior in isotopic reactions. Unlike protium, deuterium engages in reactions with a different energy profile, making it advantageous in certain contexts, especially in nuclear fusion processes.
Nuclear fusion, the process that powers the sun and other stars, involves the amalgamation of light nuclei to form a heavier nucleus, releasing immense amounts of energy. Deuterium plays an integral role in fusion reactions due to its favorable reaction cross-section. In the Fusion of deuterium with another deuterium nucleus, two primary reactions occur:
- Two deuterium nuclei can fuse to form helium-3 (³He) and a neutron (n), or
- They can produce tritium (³H) and a proton (p).
These reactions are particularly compelling for nuclear fusion research. The resultant helium-3 and tritium are themselves viable fuels for future fusion reactors, allowing for a sustainable energy source that could potentially meet global energy demands without the harmful byproducts associated with fossil fuels.
One of the alluring aspects of deuterium is its abundant presence in nature. Approximately 0.0156% of all hydrogen in seawater exists as deuterium. This availability makes it a practical resource for nuclear energy production, particularly in the context of fusion. International efforts, such as the ITER project, aim to harness deuterium and tritium fusion to create a viable form of controlled nuclear fusion on Earth.
In addition to its utilization in fusion, deuterium is essential in certain types of nuclear fission experiments. Fission, the process where a heavy nucleus splits into two lighter nuclei, releases energy in a manner distinct from fusion. In nuclear fission reactors, deuterium can serve as a moderator to slow down neutrons, facilitating a more controlled reaction. Such experiments are crucial in the development of advanced reactor designs that prioritize safety and efficiency.
Deuterium is also employed in the production of heavy water (D₂O), a form of water that contains deuterium in place of regular hydrogen. Heavy water is an integral part of certain types of nuclear reactors, such as those utilizing heavy water as a moderator. The presence of deuterium slows down neutrons without capturing them, enhancing the efficiency of the nuclear reaction. Countries like Canada, which utilize CANDU reactors, have successfully integrated heavy water technology, showcasing its efficacy in generating power sustainably.
An intriguing aspect of deuterium is its role in scientific research beyond nuclear energy. In the realm of chemistry, deuterium is used in isotopic labeling, a technique that helps scientists trace the pathways of chemical reactions. By substituting hydrogen with deuterium in a compound, chemists can gain insights into reaction mechanisms that would otherwise remain obscure. This has applications ranging from pharmacology to biochemistry, where understanding molecular dynamics is critical.
The phenomenon of deuterium also piques interest from a broader perspective. In cosmological studies, deuterium serves as a stellar probe. The primordial abundance of deuterium in the universe offers clues about the conditions of the early universe, particularly the processes that occurred during the Big Bang nucleosynthesis. When analyzing the cosmic microwave background radiation, scientists can determine cosmic parameters that shed light on the formation and evolution of the cosmos.
Throughout the diverse applications of deuterium, its isotopic distinction lends to a tapestry of scientific intrigue. The interplay between energy production, astronomical research, and chemical investigation underscores the versatility of this isotope. Nevertheless, the continued exploration of deuterium’s potential in nuclear fusion remains a focal point, as researchers seek to address the pivotal challenge of providing a clean, safe energy source for future generations.
In conclusion, deuterium, with its unique nuclear properties and multifaceted applications, occupies an essential position in both the realms of nuclear science and broader research. Its presence in fusion reactions, fission reactors, and chemical investigations exemplifies its significance in contemporary science. As the pursuit of sustainable energy continues, the exploration of deuterium’s capabilities will undoubtedly remain a cornerstone in the quest for innovation and discovery in nuclear physics.