In the realm of chemistry, the concept of atoms plays a pivotal role. Each element on the periodic table is composed of atoms, yet the questions surrounding whether these atoms are chemically bonded—both within individual elements and between elements—invites significant contemplation. This article will explore the intricate nature of atomic bonding and its implications for the composition of elements in detail.
At its core, a chemical bond refers to the attractive forces that hold atoms together in compounds or within elemental structures. Within a singular element, the behavior of atoms can vary enormously depending on the type of element in question. To understand if atoms are chemically bonded in any given element, one must consider the three primary states of matter—solids, liquids, and gases—and their underlying atomic arrangements.
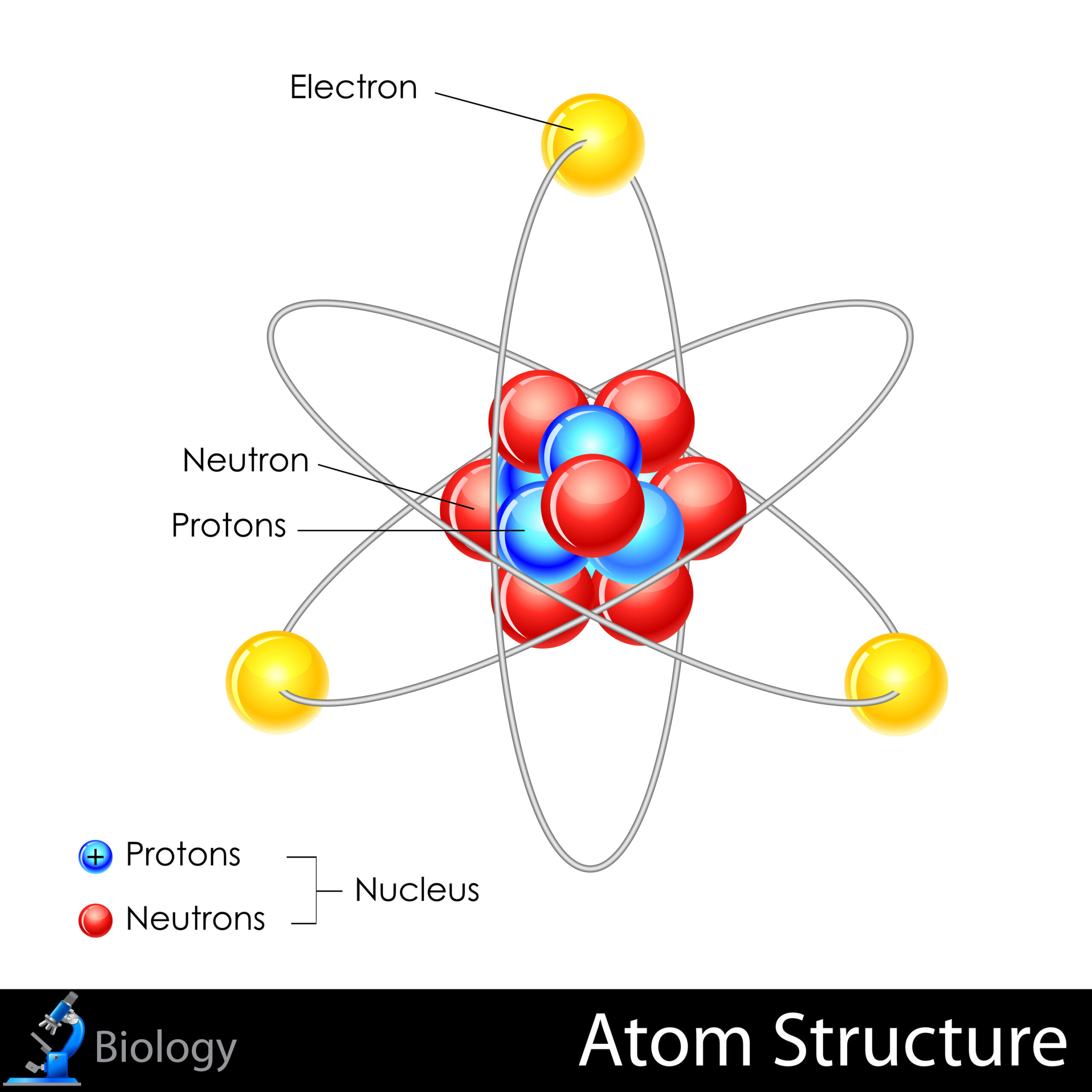
1. Atomic Structure
Atoms, the fundamental building blocks of matter, are constituted of three primary particles: protons, neutrons, and electrons. The nucleus, composed of protons and neutrons, is encircled by a cloud of electrons. This arrangement is critical when examining how atoms interact within an element. For instance, metals exhibit a different bonding behavior than nonmetals, as metallic bonding creates a “sea of electrons” that allows for conductivity and malleability.
2. Intramolecular Bonds in Elements
To ascertain whether atoms in an element are chemically bonded, one must analyze the nature of intramolecular bonds. These are the bonds that hold atoms together within a molecule. Elements such as oxygen and nitrogen exist as diatomic molecules, where two atoms share electrons to achieve stability through covalent bonding. This bonding results in the formation of O2 and N2 molecules, thereby illustrating that, indeed, within these elements, atoms are chemically bonded.
3. Atomic Bonding in Metals
Metals, on the other hand, present a different paradigm of bonding. In a metallic lattice, atoms are arranged in a structured pattern, where the valence electrons are not fixed to any single atom but are delocalized. This unique bonding leads to various properties such as electrical conductivity and ductility. Due to the strong forces of attraction between positively charged metal ions and the delocalized electrons, one can affirm that atoms in a metallic element are bonded together, creating a cohesive structure that is typically rigid yet can be reshaped under stress.
4. Allotropes and Bonding Variants
It is noteworthy that many elements can exist in multiple structural forms known as allotropes. For example, carbon can be found in forms such as diamond, graphite, and graphene. These allotropes exhibit vastly different properties due to variations in bonding patterns. In diamond, each carbon atom forms four strong covalent bonds in a tetrahedral lattice, giving rise to its hardness. Conversely, in graphite, the carbon atoms are bonded in layers, allowing them to slide past one another, resulting in lubricating properties. This highlights that while the atoms in allotropic forms are indeed bonded, the nature and strength of these bonds can vary dramatically based on the structural characteristics of the allotrope. Thus, within carbon’s allotropes, one can observe the versatility of atomic bonding and its implications for material properties.
5. Implications of Atomic Bonding
Understanding atomic bonding within elements is crucial not only in chemistry but also in materials science, biology, and even engineering. The innate properties derived from atomic arrangements and bonding types underpin the functionality of numerous materials. For instance, molecular bonding in water facilitates its role as a solvent, crucial for life’s biochemical reactions. Similarly, the covalent bonding in organic compounds allows for the diverse chemistry that forms the basis of biological organisms.
6. Quantum Mechanics and Chemical Bonds
Delving deeper into the atomic world, quantum mechanics provides an insightful lens through which to examine chemical bonding. The behavior of electrons in atoms can be described using quantum states, which dictate how atoms bond—primarily through overlapping electron clouds, sharing, or transferring electrons. The nuances involved in electron configurations and their respective energies directly influence the strength and type of bonds formed within an element. As such, a proper understanding of atomic behavior grounded in quantum mechanics profoundly enhances our comprehension of elemental bonding.
7. Conclusion
Ultimately, atoms within an element are chemically bonded, but the complexities of those bonds vary according to the element’s nature. From metallic bonds in solid metals to covalent bonds in diatomic molecules, the interplay of atomic forces shapes not only the elemental landscape but also the manifold characteristics and applications across various scientific disciplines. Future explorations in atomic interactions will likely yield even deeper insights into the nature of matter itself, further emphasizing the significance of understanding the bonds between atoms in every element.