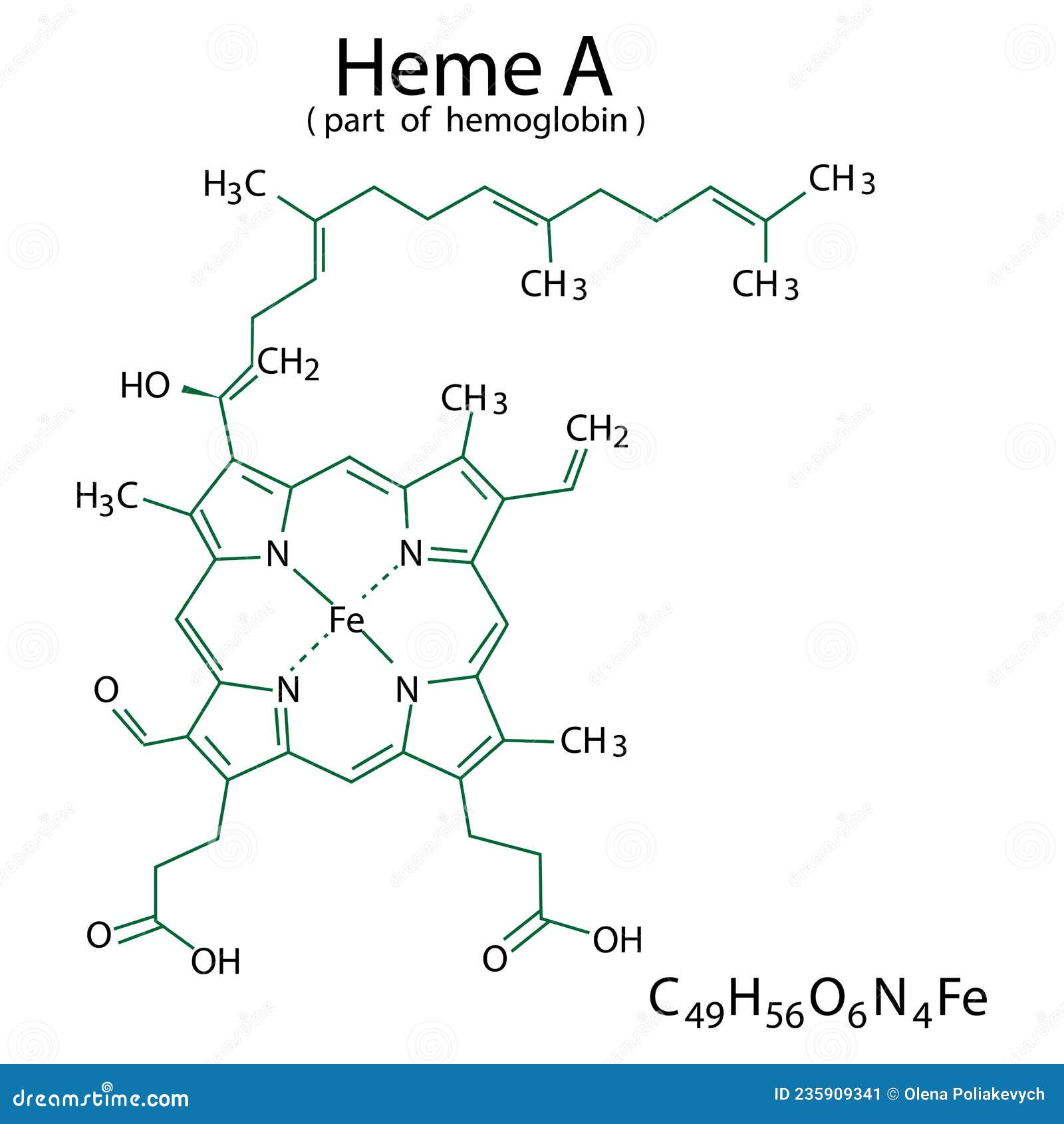
The molecular architecture of heme is a testament to the intricate nature of biochemical compounds that play pivotal roles in physiological processes. Understanding heme, particularly its molecular orbitals, can vastly transform our perspective on its functionality in biological systems. Heme, an organic compound, consists of a porphyrin ring and an iron atom, serving as a crucial component in hemoglobin, myoglobin, and various enzyme systems. This article elucidates the molecular orbitals of heme, emphasizing their significance in harnessing the oxygenation properties and reactivity of this multifaceted compound.
At its core, heme comprises a central iron (Fe) atom coordinated within a planar, cyclic tetrapyrrole structure. This porphyrin ring is a chromophore, absorbing light and giving blood its characteristic red hue. An in-depth exploration of heme’s molecular orbitals reveals layers of complexity, informing our understanding of electronic configurations and the subsequent chemical behavior of hemoproteins.
The molecular orbital (MO) theory provides a framework for examining the interactions between atomic orbitals to form new molecular orbitals. In the case of heme, the primary orbitals derived from the constituent atoms play a significant role in defining the reactivity and electronic properties of the heme group. The relevant atomic orbitals include those from carbon (C), nitrogen (N), and iron, which amalgamate to give rise to the unique MOs Central to this analysis are the π (pi) and σ (sigma) orbitals, critical for bonding and electron distribution.
Analyzing the π orbitals is essential, as they stem from the conjugated system inherent within the porphyrin structure. The delocalized π electrons across the four nitrogen-containing pyrrole units interact to produce a spectrum of molecular orbitals. These orbitals include both bonding (lower-energy) and antibonding (higher-energy) configurations. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) are of particular significance. Their energies and spatial distributions dictate the electronic transitions that occur during heme’s biochemical activity.
In the fully reduced state, the heme iron possesses a d^6 electronic configuration, firmly placing the emphasis on the interactions between the d orbitals of iron and the p orbitals of the surrounding nitrogen atoms. When heme binds to oxygen, the iron undergoes an electronic shift: this transformation engages in π-backbonding, where electron density is shared between the filled d orbitals of iron and the unoccupied π* orbitals of molecular oxygen (O₂). This interaction not only stabilizes the heme-oxygen complex but also enhances the affinity of hemoglobin for oxygen, depicting a remarkable mechanism of cooperative binding.
The geometric conformations of heme further enrich the understanding of its molecular orbitals. The planar structure accommodates sp² hybridization of carbon atoms, which synergizes with the delocalization of π electrons in conforming to the rigid architecture of the porphyrin ring. Altering this geometry through axial ligation (for instance, by nitric oxide or carbon monoxide) modulates the p-orbital interactions, significantly affecting electronic properties and reactivity. Such insights remind us of heme’s versatility in biological reactions and signal transduction pathways.
One cannot overlook the implications of substituent groups on the porphyrin core. Functional modifications at the peripheral positions of the heme structure can lead to profound alterations in the MO landscape. Substituents can influence the electron-donating or withdrawing properties, thus modulating the energy levels of the HOMO and LUMO. Through substitutions, the oxidation state of iron within the heme group can also shift, impacting its binding affinity to ligands and consequently the functional scope of hemoproteins.
To further comprehend the dynamic nature of heme’s molecular orbitals, computational chemistry has risen as a pivotal tool. Quantum mechanical models facilitate the visualization of MOs through sophisticated simulations, equipping researchers with an understanding of complex electronic behaviors. Using density functional theory (DFT), one can explore the molecular arrangements that arise when heme interacts with various ligands. Such techniques unravel the relationships between structure, electronic properties, and reactivity pathways, leading to a more nuanced understanding of biological processes.
Moreover, the investigation of heme’s molecular orbitals contributes to the wider narrative of biomimetic designs in synthetic chemistry. The principles gleaned from heme’s electronic configurations inform the creation of artificial oxygen carriers and catalysts, advancing the synthesis of therapeutic agents and materials. By leveraging knowledge of MOs, chemists aspire to replicate the precise functionalities exhibited by natural systems, paving the way for innovative solutions to enhance bioactivity and efficiency.
In summation, the exploration of heme’s molecular orbitals elucidates the sophisticated interplay between structure and function in biological molecules. The intricate orchestration of π and σ bonding, the influence of substituents, and the revelations provided by computational methodologies converge to underscore the elegance of heme’s chemical properties. This knowledge empowers scientists to venture beyond the confines of traditional biochemistry, promising a transformative understanding of the molecular underpinnings that dictate life processes. As we stride forward, it is imperative to carry these revelations into a future where chemistry continues to unravel the mysteries of molecular interactions, inspiring curiosity and innovation in the life sciences.